Reviewed by Danielle Ellis, B.Sc.Jan 4 2023
The method of single-cell profiling’ is assisting neuroscientists to note how the disease impacts significant brain cell types and determining common, possibly targetable pathways.
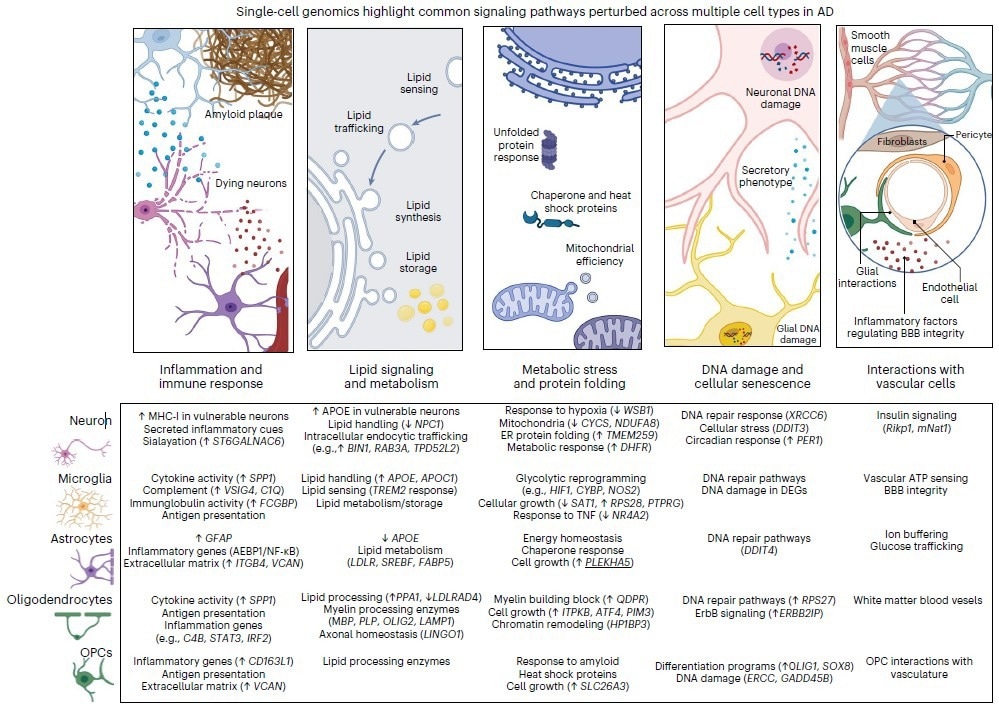 A figure from the paper depicts pathways perturbed in Alzheimer's disease. Below examples are listed for each pathway by major brain cell type. Image Credit: The Picower Institute.
A figure from the paper depicts pathways perturbed in Alzheimer's disease. Below examples are listed for each pathway by major brain cell type. Image Credit: The Picower Institute.
Following decades of basic scientific and drug discovery research, Alzheimer’s disease has stayed mysterious and hopeless, with the least number of therapeutic advances.
However, in a new review article in Nature Neuroscience, Massachusetts Institute of Technology (MIT) researchers write that by applying the new research potential of single-cell profiling, the field has quickly reached long-sought visions with powerful potential for both describing Alzheimer’s disease and doing something useful regarding it.
For example, by examining this new proof, the authors display that the disruptions converge of the disease on five major areas of cellular function, or “pathways,” in each of the five significant brain cell types.
Single-cell profiling technologies generate extensive measurements of genetic activity present in individual cells, like levels of RNA, which has been transcribed from DNA so that the functions and roles of the cell in the brain’s biology and the pathology of disease could be evaluated.
Image Credit: Juan Gaertner/Shutterstock.com
Single-cell profiling technologies tend to exceed genome sequencing, which catalogs the DNA present in almost every cell of a person. This is done by disclosing how every cell has been especially making use of that general set of instructions.
In studying Alzheimer’s disease, researchers have been making use of single-cell profiling to note how several brain cells, like diverse kinds of neurons and microglia and astrocytes, act otherwise in disease than how they conduct in a healthy brain.
In the study, MIT Brain and Cognitive Sciences doctoral student Mitch Murdock and Picower Professor Li-Huei Tsai, Director of MIT’s Picower Institute for Learning and Memory and Aging Brain Initiative, writes that while the outcomes of single-cell profiling studies verify that the terrible effects of the disease are complicated and wide-ranging, there seem to also be five pathways that turn out to be perturbed in each of five significant cell types.
The researchers write that analyzing these pathways could help produce useful biomarkers of disease and yield useful targets for therapeutic intervention:
- Inflammation and immune response
- Protein folding and metabolic stress
- Interactions with brain vasculature (blood vessels)
- Lipid (fat molecule) signaling and metabolism
- DNA damage and cellular senescence (aging)
For each of such pathways present in microglia, astrocytes, neurons, oligodendrocytes, and oligodendrocyte precursor cells, Tsai and Murdock determine particular variations in gene regulation, found in single-cell studies.
This considerably occurs in the brains of Alzheimer’s patients or mouse models than in healthy control samples.
For instance, Tsai and Murdock highlight over a dozen genes that have been all closely included in lipid processing and whose expression has been changed in several ways in various cells in the brain’s prefrontal cortex.
For another instance, they show that all five cell types display impairments in DNA repair, albeit by altered expression of various genes present in each.
“By identifying vulnerable cell types and the molecular programs that give rise to them, therapeutic interventions might reverse aberrant cellular trajectories. While many transcriptional alterations are cell-type specific, these changes ultimately might converge on shared signaling pathways across cell types that might represent targets for new therapeutic strategies,” states Murdock and Tsai in Nature Neuroscience.
To make sure, the authors have noted the fact that there is still a great deal of work to be done, both in purifying and enhancing single-cell methods and also exploiting newer related chances.
The study notes various problems that should be taken carefully into account in generating valid single-cell profiling outcomes. This includes where cells are being sampled in the brain for sequencing, from which person, and also in what condition.
Furthermore, it is not always direct to exhibit how variations in gene expression essentially tend to impact biology, and it is even harder to know if any specific intervention, for example, to target modified inflammation pathways, will prove harmless and efficient as a therapy.
At the same time, future directions can include making wider use of “spatial transcriptomics,” which helps quantify gene transcription in cells where they are located inside the brain, instead of eliminating them for analysis.
Studies must be extended to include more human samples so that altering disease and demographic variations could be fully accounted for. The authors write that it is necessary to share and combine datasets and make better comparisons between human and mouse samples. Also, it is crucial to better comprehend how well, or not, they tend to overlap.
Tsai and Murdock stated, “Single-cell profiling facilitates a nuanced portrait of the diverse cellular processes perturbed in the AD brain. These varied molecular programs help explain the divergence between healthy aging and cognitive decline, and highlight cell-type-specific molecular programs involved in AD. Core signaling modules are disrupted across multiple cell types, and manipulating disrupted cellular states will pave the way for new therapeutic opportunities.”