Reviewed by Danielle Ellis, B.Sc.Jan 4 2023
An innovative twist to the CRISPR tool—also known as “genetic scissors”—is being utilized to edit plant genomes by researchers from the Max Planck Institute of Molecular Plant Physiology, signaling a methodology change.
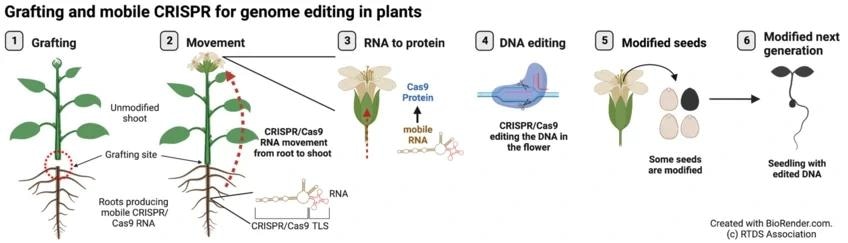 The blueprint of the CRISPR/Cas9 gene scissors is transported as RNA from the rootstock of a gene-modified plant to the grafted shoot of an unmodified plant. There, the gene scissors protein is built with the help of the RNA. The gene scissors protein edits specific genes in flowers. Plants in the next generation carry the desired gene modification. Image Credit: RTDS Association.
The blueprint of the CRISPR/Cas9 gene scissors is transported as RNA from the rootstock of a gene-modified plant to the grafted shoot of an unmodified plant. There, the gene scissors protein is built with the help of the RNA. The gene scissors protein edits specific genes in flowers. Plants in the next generation carry the desired gene modification. Image Credit: RTDS Association.
The breakthrough made could help clarify and expedite the development of novel, genetically stable commercial crop varieties by integrating grafting with a newly-developed “mobile” CRISPR tool.
An unchanged shoot has been grafted onto roots that consist of a mobile CRISPR/Cas9, enabling the genetic scissor to shift from the root into the shoot. There it helps edits the plant DNA but leaves any sign of itself in the next generation of plants.
This innovation will help save money, time, and circumvent current limitations in plant breeding and add up to bearable food solutions throughout multiple crops.
Image Credit: ART-ur/Shutterstock.com
Already, several crops that feed the world are being threatened by drought, heat, and plant pests, and these factors are being additionally exacerbated by an altering climate.
To future-proof such necessary plants for effective and efficient crop yields under difficult conditions, it is possible to edit plant genomes with high accuracy with the help of the CRISPR/Cas9 system to initiate useful gene functions or to eliminate unfavorable ones.
While CRISPR/Cas9 is known as an immense step forward for plant breeding, it remains a tedious and highly-priced solution, thereby making it impracticable for application in the majority of plants. The new development made by the research group at the Max Planck Institute of Molecular Plant Physiology in Germany defeats such limitations.
RNA as CRISPR carrier
Commercial crop plants require to be in a genetically stable manner as they lack to contain any genetic sequences collected from the CRISPR/Cas9 system and must be transgene-free. Generally, this has been achieved either via outcrossing over several generations or through dull regeneration processes.
However, both are time and money intensive and are hard, or even impossible in several crop plants. A research group headed by Friedrich Kragler set forth this to be changed. Being a part of the EU-funded PLAMORF project and the German Ministry of Research-funded proof of concept project, they are learning about transport sequences that allow the movement of RNAs from roots to shoots.
The research team determined the supposed tRNA-like sequences (TLS) that serve as signals for the long-distance movement of RNAs inside plants. The recent discovery came by integrating this breakthrough with the CRISPR/Cas9 genome editing system. While adding such a TLS to the CRISPR/Cas9 sequences, plants tend to produce “mobile” versions of CRISPR or Cas9 RNA.
Furthermore, a transgene-free, unmodified shoot has been grafted onto the roots of plants consisting of the mobile CRISPR/Cas9 RNA. This further moves from the root into the shoot and ultimately into the flowers that tend to produce the seeds.
The magic happens in the flowers. The CRISPR/Cas9 RNA moves in and is converted into the corresponding protein, which is the actual ‘genetic scissors’. It edits the plant DNA in the flowers.”
Friedrich Kragler, Max Planck Institute of Molecular Plant Physiology
Kragler added, “But the CRISPR/Cas9 system itself is not integrated in the DNA. So, the seeds that then develop from these flowers carry only the desired editing. There is no trace of the CRISPR/Cas9 system in the next generation of plants and it works with a surprisingly high efficiency.”
An editing system for many crop plants
The reason behind finding the new system highly exciting is the chance to integrate various species. The scientists displayed that “editing” in this way does not only function when the root and shoot in grafting are from the same plant species—in this case, the model plant thale cress or Arabidopsis.
Furthermore, they grafted shoots of its oilseed rape, commercial relative, onto Arabidopsis roots that produce the mobile CRISPR/Cas9. Comfortingly, the Friedrich Kragler team also discovered edited oilseed rape plants.
Our novel gene editing system can be used efficiently for many breeding programs and crop plants. This includes many agricultural important plant species that are difficult or impossible to modify with existing methods.”
Friedrich Kragler, Max Planck Institute of Molecular Plant Physiology