Reviewed by Danielle Ellis, B.Sc.Jan 6 2023
Human metabolism depends on mitochondria, which are regarded as the power plants of cells. But since 40% of the proteins in the mitochondria are dysfunctional and are linked to human diseases, mitochondria also play a significant role in medical research.
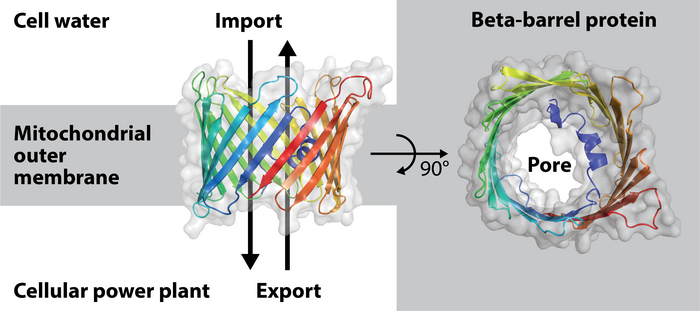 Model of the beta-barrel protein porin from baker's yeast found in the mitochondrial outer membrane. Image Credit: Christophe Wirth
Model of the beta-barrel protein porin from baker's yeast found in the mitochondrial outer membrane. Image Credit: Christophe Wirth
The formation of their barrel pores was a previously unrecognized process in the complex mitochondria. These are found inside the mitochondrial outer membrane and act as a conduit for the exchange of materials between mitochondria and the cell’s water.
Through structural and functional experiments, scientists at the Universities of Freiburg and Kyoto, Japan, have now been able to explain the guidance mechanism by which the pores are formed.
Image Credit: ART-ur/Shutterstock.com
The team’s study, which included Prof. Dr Toshiya from Kyoto Sangyo University and Prof. Drs Nils Wiedemann and Nikolaus Pfanner from the Faculty of Medicine and the CIBSS Cluster of Excellence at the University of Freiburg, was published in the journal Nature Structural & Molecular Biology.
Barrel pores are essential for life, as they are indispensable for the exchange of substances within cells. Understanding them better is an important building block for basic cellular research.”
Dr Nils Wiedemann, Faculty of Medicine, University of Freiburg
Similarities to the wine barrel structure
The barrel pores in mitochondria are made of a protein molecule that has to be folded through the outer membrane up to 19 times, as was previously known. A pore is created in the mitochondrial outer membrane as a result of the arrangement of these so-called beta-strands, which span the outer membrane and resemble the longitudinal woods/staves of wine barrels.
The sorting and assembly machinery (SAM) in the outer membrane of the mitochondria constructs beta-barrel proteins.
Roles of Sam50 and Sam37
It was established that the final beta-strand of beta-barrel proteins is initially attached to Sam50 (a protein subunit of the sorting and assembly machinery) in the mitochondrial outer membrane by purifying and elaborating for the first time a protein complex structure of the sorting and assembly machinery together with a beta-barrel protein during beta-barrel formation.
The remaining strands are then put together one by one. The portion of the beta-barrel proteins’ protein chain that is upstream of the strands in this process is crucial for both their assembly and function.
The beta-barrel protein strands of the Sam37 subunit are arranged in a circular pattern around a protrusion that extends into the mitochondrial outer membrane. The protrusion of Sam37 was shown to be essential for ring closure of the beta-barrel proteins in functional experiments where it was removed.
Dr Wiedemann added, “This allowed us to assign the Sam37 subunit a function as a cooper/barrel maker for the formation of the vital beta-barrel membrane proteins in our cellular power plants.”
Source:
Journal reference:
Takeda, H., et al. (2022). Mitochondrial sorting and assembly machinery operates by β-barrel switching. Nature Structural & Molecular Biology. doi.org/10.1038/s41586-020-03113-7