A study team headed by Dr Yuanliang Zhai from The University of Hong Kong’s (HKU) School of Biological Sciences and his associates from The Hong Kong University of Science and Technology (HKUST) and Institut Curie in France discovered a new mechanism of the human MCM2-7 complex in controlling replication initiation, which can be employed as a unique and effective anticancer method with the potential for selective death of cancer cells.
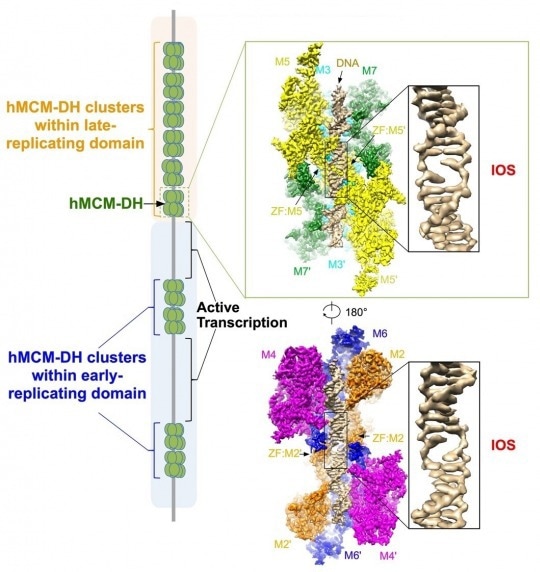 An initial open structure (IOS) is formed upon binding of human MCM double hexamer (hMCM-DH) to origin DNA. Image Credit: Image modified from the original illustration of Li, et al., 2023, Cell.
An initial open structure (IOS) is formed upon binding of human MCM double hexamer (hMCM-DH) to origin DNA. Image Credit: Image modified from the original illustration of Li, et al., 2023, Cell.
The research was published in the journal Cell.
Human life begins with a single fertilized egg in the womb of the mother. This egg divides into cells and matures into the multicellular body. Human genome DNA, the blueprint of genetic information, is precisely copied during each cell division.
Every cell has around 2 m of DNA organized into 23 pairs of chromosomes. The human body synthesizes more than a light year’s length of DNA of ~1016 m—the distance traveled by light in one year—during one’s lifetime (~70 years).
The replication process necessitates first melting the DNA duplex and then separating it into two single-stranded templates for DNA polymerases to synthesize as complement strands. Any disruption in this pathway might have serious effects, including cancer and inherited genetic diseases.
Unlocking the secret of DNA replication is key to understanding the mystery of life. Solving the structures of replication machines is central to inform their molecular functions as seeing is believing.”
Yuanliang Zhai, Assistant Professor, School of Biological Sciences, University of Hong Kong
How DNA duplexes are originally melted has been a long-standing topic for scientists since 1953, when James Watson and Francis Crick discovered the structure of DNA. The enzyme responsible for unzipping DNA duplex during replication in eukaryotes was discovered in 1983 by research collaborator Professor Bik-Kwoon TYE at Cornell University as the minichromosome maintenance protein complex (MCM) genes from wine brewing yeast.
As the catalytic core of the DNA replicative helicase, the six MCM genes MCM2 to MCM7 (MCM2-7) produce a six-subunit ring complex. To begin DNA replication in organisms, the MCM2-7 complex must first be formed into a head-to-head double hexamer (DH) surrounding duplex DNA at thousands of locations along each chromosome.
Only a subset of the assembled MCM2-7 DHs will be eventually selected and turned into effective replicative helicases for DNA unwinding. It was thought that MCM2-7 DH may directly destabilize DNA in order to cause an initial opening of duplex DNA. The fundamental mechanism, however, remained largely unclear.
To respond to this question, the researchers used cryo-electron microscopy (cryo-EM), a cutting-edge technology, to visualize the atomic features of the MCM2-7 DH, which are millions of times smaller than the resolution limit of human eyes.
As reported in the journal Nature in 2015, the scientists successfully solved the first cryo-EM structure of MCM2-7 DH isolated from yeast at 3.8 Å. Unfortunately, the acquired DNA was unstable and did not provide information about the status of the MCM2-7 DH-bound DNA duplex. The MCM2-7 DH was successfully extracted from cultured human cells and its structure was discovered at 2.59 Å.
This high-resolution structure shows how the MCM2-7 complex destabilizes DNA, resulting in an initial opening of the DNA duplex precisely at the intersection of the two connected MCM2-7 hexamers.
The researchers also discovered that the MCM2-7 DHs are loaded onto DNA at tens of thousands of locations across the human genome that are mutually exclusive with active transcription loci. Moreover, when this initial open structure is disrupted, MCM2-7 DHs are unable to assemble onto DNA, thus suppressing DNA replication initiation.
The atomic-resolution cryo-EM structures enabled direct visualization of the initial DNA melting, which is crucial for us to understand the molecular mechanism of DNA replication. This study also demonstrates the importance of collaboration. Efforts from research groups with complementary expertise are required to answer the fundamental biological questions.”
Dr Shangyu Dang, Assistant Professor, Division of Life Science, Hong Kong University of Science and Technology
Several cancer therapeutic drugs have targeted DNA replication. However, existing medications destroy all dividing cells indiscriminately since both normal and malignant cells require DNA replication for cell growth. As a result of their specificity, these anticancer chemotherapies create major issues.
Inhibiting DNA replication start is a more preferable method since it will cause cancer cells to go through apoptosis while normal cells will be stopped in the G1 phase (first growth phase) or withdraw from the cell cycle into the G0 state (resting phase). As a result, inhibiting replication start can be used as a unique and effective anticancer treatment with the potential for cancer cell selective death.
The results of this study offer high-resolution structural and mechanistic details on the human pre-initiation complex that may one day be used to create safe anticancer medications that employ the MCM2-7 complex as a target.
Source:
Journal reference:
Li, J., et al. (2023) The human pre-replication complex is an open complex. Cell. doi.org/10.1016/j.cell.2022.12.008.