Cells zealously protect the integrity of their genomes, because damage can lead to cancer or cell death. The genome—a cell’s complete set of DNA—is most vulnerable while it is being duplicated before a cell divides. Cancer cells are dividing constantly, so their genomes are constantly in jeopardy.
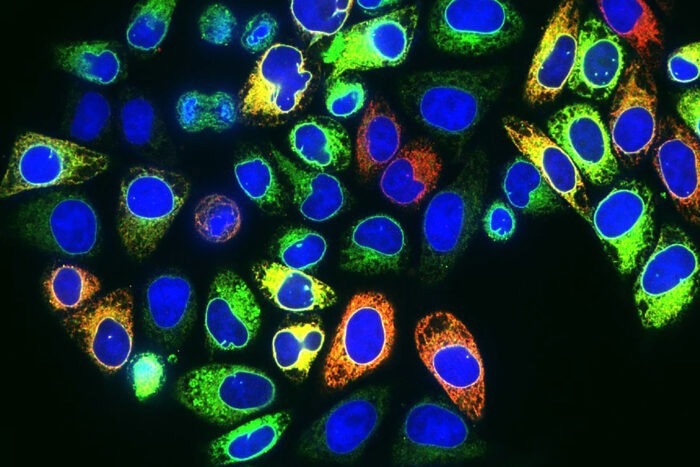 Proteins labeled with colored tags fill the main compartment—but not the nuclei (blue)—of human cervical cancer cells. Green cells contain the protein TRPV2, red cells contain STING, and yellow and orange cells contain a mixture of both. The proteins are part of a newly discovered DNA-protection pathway that potentially could be targeted to improve cancer therapies, according to researchers at Washington University School of Medicine in St. Louis. Image Credit: LINGZHEN KONG
Proteins labeled with colored tags fill the main compartment—but not the nuclei (blue)—of human cervical cancer cells. Green cells contain the protein TRPV2, red cells contain STING, and yellow and orange cells contain a mixture of both. The proteins are part of a newly discovered DNA-protection pathway that potentially could be targeted to improve cancer therapies, according to researchers at Washington University School of Medicine in St. Louis. Image Credit: LINGZHEN KONG
A previously undiscovered signaling route that cells utilize to safeguard their DNA as it is being duplicated has been discovered by researchers at Washington University School of Medicine in St. Louis.
Targeting this system could increase the effectiveness of cancer therapeutics, according to the results, which were published on January 24th, 2023 in the journal Molecular Cell.
A cell that can’t protect its genome is going to die. This entire pathway we found exists to protect the genome so the cell can survive in the face of replication stress. By combining inhibitors of this pathway with chemotherapy drugs that target the DNA replication process, we potentially could make such drugs more effective.”
Zhongsheng You, PhD, Professor, Cell Biology and Physiology, Department of Cell Biology and Physiology, Washington University School of Medicine in St. Louis
Replication stress happens when there are issues with the cell’s DNA duplication machinery when duplicating the genome. Since they contain a high number of repetitive sequences, some regions of DNA are naturally difficult to replicate.
Replication stress is also brought on by factors that harm DNA, such as radiation and toxic chemicals, and also by the activation of genes that cause cancer.
Numerous cancer treatments, including well-known agents like doxorubicin and cisplatin, function by altering DNA and raising replication stress.
You investigates how cells defend their genomes during replication. He focused on the ATR-Chk1 genome-protection pathway early in his career. This route regulates the cell-division cycle and inhibits stalled replication machinery from failing completely and leading to DNA breakage.
He and his team have been carefully stitching together another previously unidentified genome-protection route for the past eight years. The last piece of the puzzle has been put together with the help of this recent study.
The mechanism they uncovered works as follows: A protein called Exo1 that typically trails after the machinery gets a bit out of control when the DNA-duplicating machinery slows.
Exo1 is responsible for performing quality control by removing badly duplicated DNA, but when the machinery stalls, Exo1 begins randomly chopping away, cleaving off chunks of DNA that eventually escape the nucleus and enter the cell’s interior.
Under normal circumstances, DNA cannot be detected beyond the nucleus, hence its discovery outside the body of the cell raises a red flag. A sensor molecule that detects DNA fragments sets off a series of chemical reactions, including the release of the calcium ion from the endoplasmic reticulum, a cellular organelle.
This causes Exo1 to shut down and stop further genome slicing until the machinery issue is resolved.
According to this recent study, the finding of DNA fragments acts as a warning flag, triggering the entire genome-protection response. First author Shan Li, PhD, led the investigation while working as a postdoctoral researcher in You’s lab and later as a staff scientist.
Li is currently an assistant professor at Zhejiang University School of Medicine in Hangzhou, China. Graduate student Lingzhen Kong, who is a co-author, also made significant contributions to the study.
Eight protein factors that are involved in this pathway for genome protection have been discovered by You and his colleagues over time. The majority of them already have inhibitors in the works that could be used in cancer research.
You stated, “Now that we have the pathway, we want to know whether it can be targeted for cancer treatment. Lung, ovarian, and breast cancer are intrinsically under replication stress. Other cancers are put under replication stress by chemotherapy drugs. This pathway protects cells from replication stress, so if we could block the pathway, it might improve patients’ response to cancer therapies.”
Numerous proteins involved in this pathway also play crucial roles in other biological processes, including immunity, metabolism, and autophagy, which is the process by which cells break down waste materials.
“One of the most exciting things about this pathway is how it intersects with so many other pathways. I have been focusing on cancer, but much of this could also apply to autoimmune diseases. Two of the proteins we identified have been linked to chronic activation of the immune response and autoimmune disease,” further added You.
He concluded, “We want to understand the relationship between this replication-stress response pathway and the innate immune response pathway. The work we do is very basic, and it is so exciting to connect the dots between these fundamental processes and see how they relate to human health and disease.”
Source:
Journal reference:
Li, S., et al. (2023). Cytosolic DNA sensing by cGAS/STING promotes TRPV2-mediated Ca2+ release to protect stressed replication forks. Molecular Cell. doi.org/10.1016/j.molcel.2022.12.034