Nucleic acid-targeting photodynamic therapy (PDT) is an innovative type of targeted therapy that is currently being studied. This treatment is based on photosensitizers, which are drugs that attach to specific regions in a cell’s DNA.
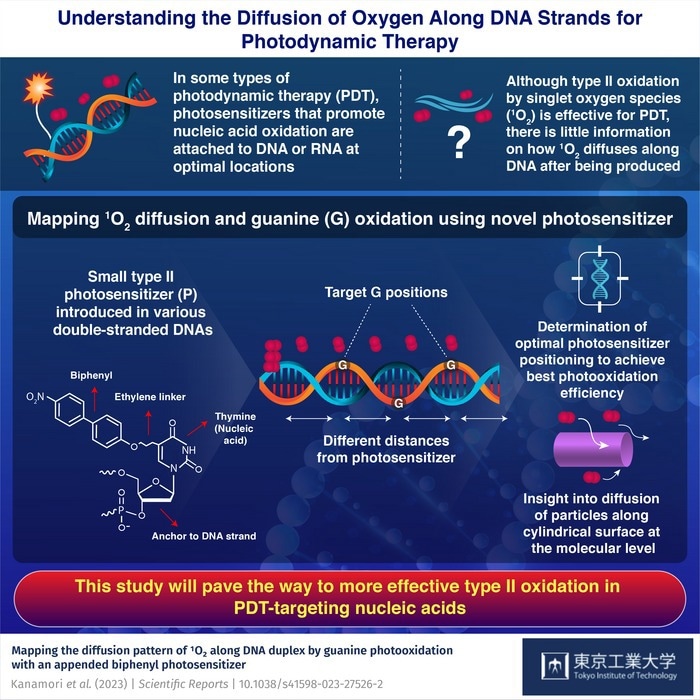 Mapping the diffusion pattern of 1O2 along DNA duplex by guanine photooxidation with an appended biphenyl photosensitizer. Image Credit: Tokyo Institute of Technology.
Mapping the diffusion pattern of 1O2 along DNA duplex by guanine photooxidation with an appended biphenyl photosensitizer. Image Credit: Tokyo Institute of Technology.
After the cells have been bound, they are irradiated at a specific frequency, which leads the photosensitizer to produce reactive oxygen species (ROS) or singlet oxygen (1O2) molecules. These chemicals oxidize neighboring nucleic acids, causing genetic damage and annihilating the irradiated cell.
Even though the entire process appears simple, there are some obstacles to overcome before this type of PDT is suitable for clinical use. One of them is that, while type II oxidation (induced by 1O2) has some benefits over type I oxidation (caused by ROS), there is limited knowledge on how far 1O2 molecules can travel once produced. Because of this knowledge gap, it is hard to determine which location in the DNA should be targeted to produce the best result.
Investigators from the Tokyo Institute of Technology, Japan, attempted to address this issue in their recent research. The researchers, guided by Professor Hideya Yuasa, used a unique approach to explore how 1O2 propagates through double strand DNA and how well it can oxidize neighboring guanine (G) sites depending on the distance to the photosensitizer. The study was published in the journal Scientific Reports.
The researchers created a series of double-stranded DNA molecules containing several G sites at various places relative to the photosensitizer’s anchoring point. They then examined which G sites were regularly oxidized after irradiating the DNA.
It is indeed worth noting that the photosensitizer they employed was based on earlier research guided by Prof. Yuasa. The photosensitizer in this case was a biphenyl group “hanging” from a short, freely rotatable linker attached to thymine, one of the DNA building blocks.
The small size of this photosensitizer, which ensured that 1O2 diffusion was not greatly affected, and its surprisingly high tendency to create 1O2 solely upon irradiation when compared to other photosensitizers, made it particularly effective for this study.
The researchers identified the optimal distances to the photosensitizer to obtain the maximum oxidation of G after multiple experiments and theoretical studies. Furthermore, they provided insight into specific electronic mechanisms that inhibit G oxidation at places closer to the photosensitizer.
Our study provides information about how 1O2 travels along DNA duplexes in more detail than ever, thereby offering clues on how to overcome the low reactivity of type II photooxidation in nucleic acid targeting PDT.”
Hideya Yuasa, Professor, Tokyo Institute of Technology
The observations of this study inch toward next-generation PDT, which could be a powerful tool in the fight against cancer.
Our mapping of the diffusion of 1O2 along DNA duplexes will be important to develop efficient and selective photosensitizer agents for PDT. It also serves as an experimental demonstration of the diffusion of particles along a cylindrical surface at the molecular level.”
Hideya Yuasa, Professor, Tokyo Institute of Technology
Source:
Journal reference:
Kanamori, T., et al. (2023) Mapping the diffusion pattern of 1O2 along DNA duplex by guanine photooxidation with an appended biphenyl photosensitizer. Scientific Reports. doi.org/10.1038/s41598-023-27526-2.