The accumulation of amyloid beta proteins, which encourage synaptic malfunction, causes cognitive decline in Alzheimer’s disease (AD), according to scientific evidence. One of the neuropathological features of AD brains is the degeneration of basal forebrain cholinergic neurons, which leads to a reduction in the number of cholinergic projections to the hippocampus.
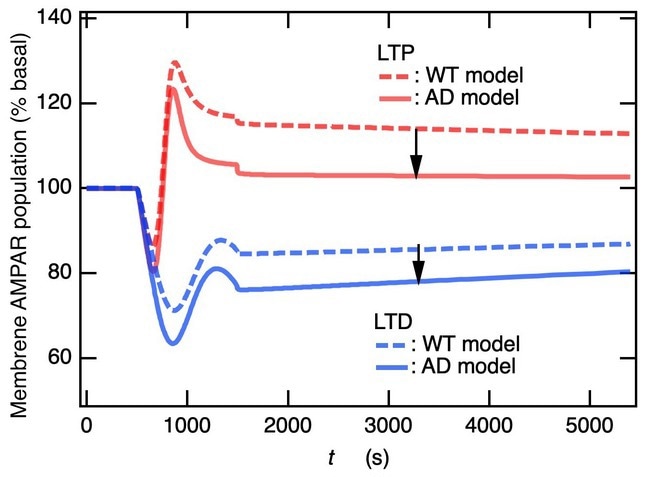 The graph shows the involvement of the percentage of basal membrane AMPAR population in long-term potentiation and differentiation in wild type and Alzhiemer’s disease animal models. The study demonstrates that M1-mAChR- and NMDAR-dependent long-term potentiation and differentiation share a common pathway. Image Credit: Tomonari Sumi and Kouji Harada from Okayama University
The graph shows the involvement of the percentage of basal membrane AMPAR population in long-term potentiation and differentiation in wild type and Alzhiemer’s disease animal models. The study demonstrates that M1-mAChR- and NMDAR-dependent long-term potentiation and differentiation share a common pathway. Image Credit: Tomonari Sumi and Kouji Harada from Okayama University
Some drugs widely recognized as acetylcholinesterase inhibitors are used to improve cholinergic neurotransmission as a symptomatic treatment for AD. Understanding how acetylcholine controls synaptic transmissions is critical for better prevention and treatment of cognitive disorders such as AD and schizophrenia.
Signaling through the M1 muscarinic acetylcholine receptor (mAChR) regulates higher brain functions such as learning and memory. Long-term potentiation (LTP) and long-term depression (LTD) of excitatory synaptic transmission are also induced by the mAChR in the hippocampus.
Extracellular levels of acetylcholine (Ach) in the hippocampus increase fourfold during hippocampus-controlled learning activities, owing to mAChR signal transmission. Agonists (activator chemicals) have been shown to stimulate LTP and LTD in the hippocampus, but the molecular mechanisms underlying are unknown.
To investigate these molecular mechanisms, scientists from Japan recently created a model to monitor hippocampal synaptic plasticity. Their study was published in the March 17th, 2023, issue of iScience.
Here, we propose the hypothesis that M1 mAChR-dependent LTP and LTD share the common a-amino-3-hydroxy5-methyl-4-isoxazolepropionic acid receptor (AMPAR) trafficking pathway associated with NMDAR dependent LTP and LTD.”
Tomonari Sumi, Associate Professor, Okayama University
To induce N-methyl-D-aspartate receptor (NMDAR)-dependent synaptic plasticity in hippocampal neurons, an AMPA receptor (AMPAR) trafficking model was developed. The results of this study confirmed the hypothesis that mAChR-dependent LTP and LTD share a similar AMPAR trafficking pathway.
The distinction between the two pathways is that Ca2+ ions retained in the endoplasmic reticulum of neurons are released into the spine cytosol during M1-mAChR activation. LTP and LPD are regulated by competition between Ca2+ dependent exocytosis and endocytosis.
Therefore, it can be concluded that the M1 mAChR-dependent induction of LTP and LTD shares the common AMPAR trafficking pathway with NMDAR-dependent synaptic plasticity, and new gene expression is not necessary, at least in the early stages of LTP and LTD.”
Kouji Harada, Center for IT-Based Education, Toyohashi University of Technology
The observations demonstrate how variable gene expression levels impact the induction of LTP and LTD by reducing the number of AMPARs. These findings will help researchers comprehend the factors that influence LTP and LTD in animal models of AD, which will eventually aid in the development of AD therapies that target synaptic plasticity in humans.
The expression of a number of neurotransmitter receptors, such as GluA1, which stimulates the integration of AMPA receptors inside synaptic membranes, decreases significantly as the human brain ages. The AMPAR trafficking model suggests that the changes in LTP and LTD seen in AD may be due to an age-related decrease in AMPAR expression levels.
Taken together, these observations suggest that either upregulation of neurotransmitter receptor genes or suppression of the downregulation could improve synaptic dysfunction during AD.”
Tomonari Sumi, Associate Professor, Okayama University
Source:
Journal reference:
Sumi, T. & Harada, K. (2023) Muscarinic acetylcholine receptor-dependent and NMDA receptor-dependent LTP and LTD share the common AMPAR trafficking pathway. iScience. doi.org/10.1016/j.isci.2023.106133.