Reviewed by Danielle Ellis, B.Sc.Apr 7 2023
Carbon nanotubes (CNTs) are novel nanomaterials with potential uses in a variety of sectors. However, the harm they represent to people is unknown, and studies have revealed that multiwalled CNTs cause an immune response in mice.
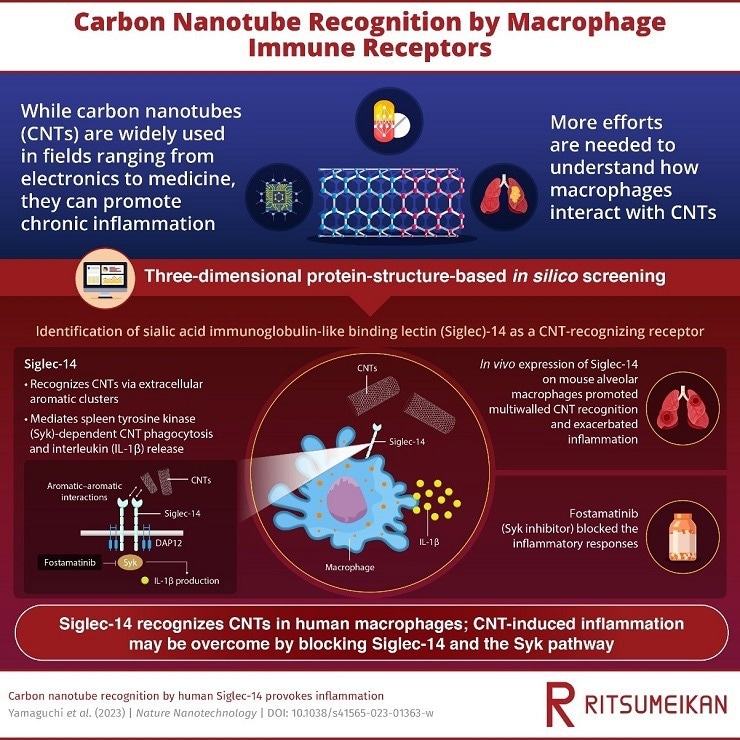
Image Credit: Ritsumeikan University
Researchers in Japan have now discovered that “Siglec-14” receptors on macrophages have a role in the detection of CNT in humans. Their study fills in some crucial gaps about the cellular processes underpinning the interactions between biological systems and CNTs.
Carbon nanotubes (CNTs) are now a cornerstone of the nanotechnology industry. CNTs have recently attracted a lot of attention from researchers due to their novel uses in materials science, electronics, and medicine.
CNTs have now been marked on the “Substitute It Now!” database of chemicals that are likely to have their usage limited, according to the International Chemical Secretariat (ChemSec). In fact, ChemSec has suggested that adequate studies of the danger of CNTs to human health are urgently needed due to their permanence in nature and possible toxicity to people.
Image Credit: urfin/Shutterstock.com
Similar to asbestos, CNTs are targeted by the immune system after entering the body and are then preferentially ingested by macrophages.
As demonstrated in rodent models, the subsequent inflammatory response, which includes the release of inflammasome and interleukin-1β (IL-1β), can lead to chronic inflammation, fibrosis, and even mesothelioma. Unfortunately, it is yet unclear how human macrophages identify CNTs.
Researchers from Japan have now shown that human macrophages recognize CNTs through the extracellular receptors Siglec-5 and Siglec-14, which are sialic acid immunoglobulin-like binding lectins.
The study team, which includes Ritsumeikan University Professor Masafumi Nakayama, expanded on earlier findings that demonstrated how mouse T-cell mucin immunoglobulin 4 (Tim4) receptors linked to CNTs via an aromatic-aromatic interface.
The results of this study have been reported in Nature Nanotechnology.
We hypothesized that aromatic clusters were essential to CNT recognition. But at the time, it wasn't evident that Tim4 receptors had a role in CNT engulfment by human macrophages.”
Masafumi Nakayama, Professor, Ritsumeikan University
To confirm how the aromatic rings enabled the Siglec-CNT interaction, the team employed three-dimensional protein structure-based in silico screening and ectopic in vivo expression of Siglec-14 receptors. The researchers identified the link between aromatic residues on Siglec-5’s extracellular loop and CNTs using molecular dynamics simulations.
On the other hand, they discovered that Siglec-14 triggered macrophage production of IL-1 β and spleen tyrosine kinase (Syk) pathway-dependent phagocytosis of CNTs. Importantly, the study discovered that this interaction significantly worsened pulmonary inflammation.
The scientists also observed that the identification of CNTs was improved in mouse macrophages that expressed human Siglec-14.
Last but not least, the Syk inhibitor medication fostamatinib was shown to suppress the Siglec-14-mediated proinflammatory signal cascade.
While elaborating on the significance of the teams’ work, Prof. Nakayama added, “This is an encouraging result as this drug may overcome CNT-induced inflammation.”
Despite their excitement at their discovery, the team is careful to point out that their results do not necessarily imply that CNTs are as dangerous as asbestos.
The method of exposure, the dosage of CNTs ingested, and the sizes and shapes of the CNTs in question will all have an impact on how poisonous CNTs are estimated to be. To correctly assess the risk presented to humans at this point, further thorough investigations are needed.
Prof. Nakayama, however, is pleased that his team has set the stage for creating safer CNTs.
He concluded, “Even if CNTs are likely to cause inflammatory diseases, our findings will help develop novel therapies, like anti-Siglec-14 monoclonal antibodies and fostamatinib, to prevent such conditions.”
Source:
Journal reference:
Yamaguchi, S. I., et al. (2023). Carbon nanotube recognition by human Siglec-14 provokes inflammation. Nature Nanotechnology. doi.org/10.1038/s41565-023-01363-w