Reviewed by Danielle Ellis, B.Sc.Apr 28 2023
Human lifespan is associated with the aging of individual cells. Three years ago, a group of researchers from the University of California, San Diego, uncovered fundamental mechanisms underlying the aging process. After identifying two separate pathways that cells take during aging, the researchers genetically altered these pathways to lengthen cell longevity.
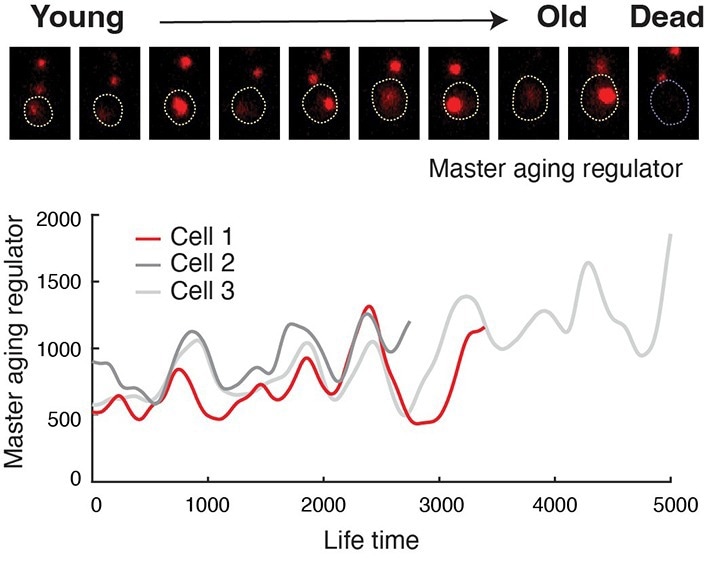
Engineered cells show oscillating abundance of a master aging regulator. Image Credit: Hao Lab, UC San Diego.
They have now extended their research using synthetic biology to build a solution that prevents cells from attaining their normal levels of degeneration associated with aging, as published in Science on April 28th, 2023. Cells, including yeast, plants, animals, and humans, all have gene regulatory circuits that control various physiological activities, including aging.
These gene circuits can operate like our home electric circuits that control devices like appliances and automobiles.”
Nan Hao, Study Senior Author and Professor, Department of Molecular Biology, School of Biological Sciences, University of California, San Diego
Nan Hao is also the co-director of UC San Diego’s Synthetic Biology Institute.
The UC San Diego team discovered, however, that under the guidance of a central gene regulatory circuit, cells do not necessarily age in the same way. Consider a car that ages when either the engine or the transmission wears out, but not both at the same time. The researchers at UC San Diego imagined a “smart aging process” that extends cellular longevity by cycling degeneration from one aging mechanism to another.
The researchers in the latest study genetically rewired the circuit that governs cell aging. From its normal role as a toggle switch, researchers created a negative feedback loop to slow the aging process. The rewired circuit functions as a clock-like device called a gene oscillator, which causes the cell to cycle between two damaging “aged” states on a regular basis, avoiding extended commitment to either and therefore decreasing the cell’s degeneration.
These breakthroughs resulted in a significantly increased cellular lifespan, setting a new record for life extension via genetic and chemical interventions.
 Image Credit: Anusorn Nakdee/Shutterstock.com
Image Credit: Anusorn Nakdee/Shutterstock.com
The investigators started with computer simulations of how the core aging circuit works, as many electrical engineers do. This aided them in designing and testing concepts before constructing or altering the circuit in the cell. When compared to more traditional genetic strategies, this approach saves time and resources in identifying effective pro-longevity strategies.
This is the first time computationally guided synthetic biology and engineering principles were used to rationally redesign gene circuits and reprogram the aging process to effectively promote longevity.”
Nan Hao, Study Senior Author and Professor, Department of Molecular Biology, School of Biological Sciences, University of California, San Diego
An interdisciplinary UC San Diego research team began exploring the mechanics of cell aging several years ago, a complex biological process that underpins human longevity and many diseases. They discovered that cells go through a series of molecular changes during their lifetimes until they deteriorate and die.
However, they discovered that cells with the same genetic material and in the same environment can age in different ways. About half of the cells age due to a gradual decline in the stability of DNA, which stores genetic information. The other half ages in a way that is linked to the decline of mitochondria, which are the energy-generating units of cells.
The latest synthetic biology breakthrough has the potential to reshape scientific approaches to aging. Unlike previous chemical and genetic attempts to force cells into artificial states of “youth,” the new research shows that slowing the ticks of the aging clock is possible by actively preventing cells from committing to a pre-destined path of decline and death, and clock-like gene oscillators could be a universal system to accomplish this.
“Our results establish a connection between gene network architecture and cellular longevity that could lead to rationally-designed gene circuits that slow aging,” the scientists note in their research.
During their research, the team looked at Saccharomyces cerevisiae yeast cells as a model for human cell aging. They created and used microfluidics and time-lapse microscopy to study the aging processes of cells.
In the current work, yeast cells that were synthetically rewired and aged under the supervision of a synthetic oscillator device had an 82 percent longer lifespan than control cells that aged normally. The findings showed “the most pronounced lifespan extension in yeast that we have observed with genetic perturbations,” they added.
Our oscillator cells live longer than any of the longest-lived strains previously identified by unbiased genetic screens.”
Nan Hao, Study Senior Author and Professor, Department of Molecular Biology, School of Biological Sciences, University of California, San Diego
“Our work represents a proof-of-concept example, demonstrating the successful application of synthetic biology to reprogram the cellular aging process, and may lay the foundation for designing synthetic gene circuits to effectively promote longevity in more complex organisms,” the authors noted.
The team is now expanding its research to include the aging of various human cell types, such as stem cells and neurons.
Cell aging oscillations
A movie of an engineered cell that ages with oscillating abundance of a master aging regulator. Video Credit: Hao Lab, UC San Diego
Source:
Journal reference:
Zhou, Z., et al. (2023) Engineering longevity—design of a synthetic gene oscillator to slow cellular aging. Science. doi.org/10.1126/science.add7631.