Researchers across the world are in search of ways to combat bacteria that could evade the present arsenal of antibiotics. This is done as a measure to avoid a global health crisis.
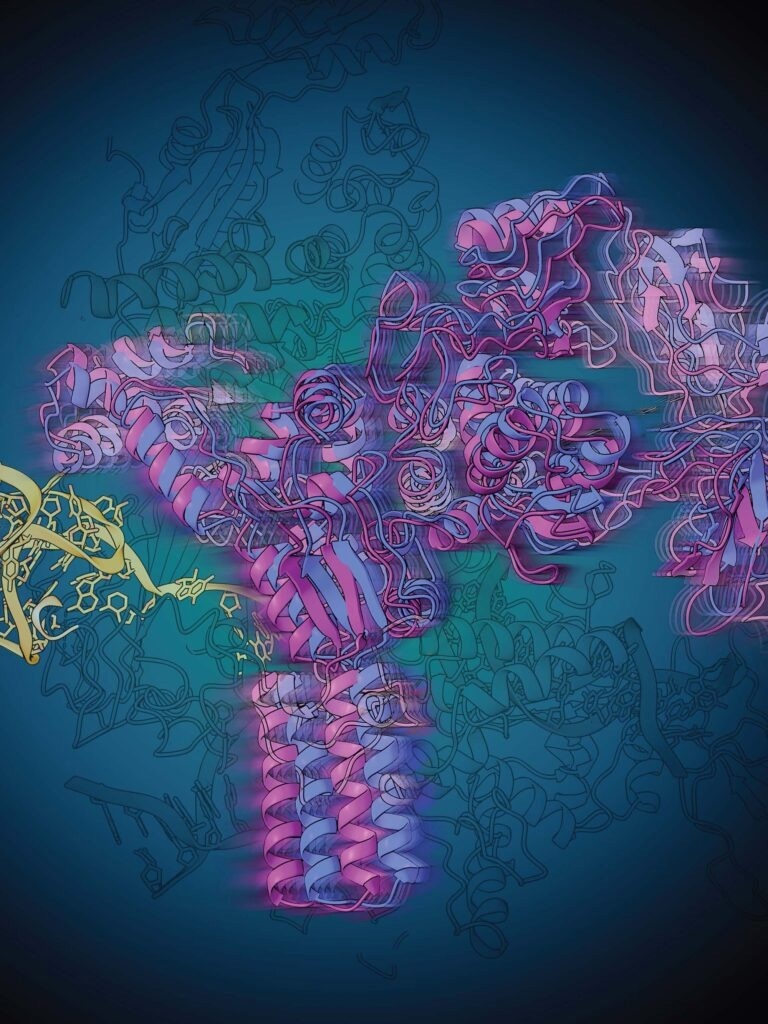 Riboswitches, small stretches of RNA that regulate a process necessary for the production of proteins by the bacterial cell, are a promising target for new and improved antibiotics. Now researchers at the University of Michigan’s Department of Chemistry and the Life Sciences Institute revealed through a powerful imaging approach how a particular riboswitch regulates its synthesis. Image credit: Rajani Arora, Life Sciences Institute
Riboswitches, small stretches of RNA that regulate a process necessary for the production of proteins by the bacterial cell, are a promising target for new and improved antibiotics. Now researchers at the University of Michigan’s Department of Chemistry and the Life Sciences Institute revealed through a powerful imaging approach how a particular riboswitch regulates its synthesis. Image credit: Rajani Arora, Life Sciences Institute
A hopeful target developed for new and improved antibiotics are riboswitches, small stretches of RNA that control a process essential for the production of proteins by the bacterial cell.
Riboswitches are discovered solely in bacteria and can be targeted with antibiotics so that animals or humans are unharmed. With a complete insight into how riboswitches work, scientists might be able to develop drugs that interrupt the cellular machinery that makes required proteins.
Currently, scientists at the University of Michigan’s Department of Chemistry and the Life Sciences Institute have disclosed the fact that utilizing a combination of biochemistry, structural biology, and computational modeling, how a specific riboswitch controls its synthesis.
The first step in producing a protein from the genetic code is known as transcription. The enzyme RNA polymerase (or RNAP) travels together with the DNA, thereby copying the genetic information discovered in DNA into a strand of RNA.
At the time of this process, RNAP will experience numerous “pauses” as it waits for additional instructions from the cell. For a long time, mechanisms for this pausing and restart have been subtle to researchers but promise to turn out to be an ideal target for antibiotics.
The research group, headed by chemistry Professor Nils Walter via a partnership with the labs of LSI professor Melanie Ohi and former U-M scientist Aaron Frank, utilized a structural biology method known as single particle cryo-electron microscopy (cryo-EM) to envision how this transcriptional regulation happens.
Their study outcomes are reported in the journal Nature Structural & Molecular Biology.
The Walter laboratory was in search of a specific riboswitch that binds a molecule made by the cell, known as preQ1. When the preQ1 molecule attaches to the riboswitch it changes the shape of the RNA, which further enables the RNAP to once again continue together with the DNA so that transcription is continued.
In 2002, riboswitches were first discovered, but their particular roles concerning the transcription machinery are yet to be understood completely. Also, it is not difficult to see why that is, states Adrien Chauvier, a Walter lab scientist and expert on riboswitches.
This is a David vs. Goliath situation, RNAP is this giant Goliath and the riboswitch is David. Because of this drastic size difference, visualizing where and how preQ1 regulates transcriptional pausing is equal to finding a needle in a haystack.”
Adrien Chauvier, Scientist, Walter Lab, University of Michigan
A previous study performed by the Walter lab disclosed that transcriptional pausing is switched on and off as a function of the preQ1 molecule which helps to bind the riboswitch. Going ahead, the Walter lab paired with cryo-EM expert Ohi to envision what was taking place.
This work is a great example of the strength of doing science at the University of Michigan. Three labs with different expertise were able to form a multidisciplinary collaboration that led to an important and novel discovery.”
Melanie Ohi, Professor, Cell and Developmental Biology, Medical School, University of Michigan
Ohi added, “These findings wouldn’t have been possible without this synergy, along with the investments the university has put into strengthening cryo-EM and RNA biology at U-M in recent years.”
Single particle cryo-EM has the potential to identify the structures of large protein complexes by constructing 3D models from millions of 2D images of particles frozen in various orientations, thereby disclosing structures that consist of molecular details that offer functional knowledge.
The structural information gathered from single particle cryo-EM corroborated the Walter lab’s previous findings but also disclosed a particular change in the shape of the riboswitch that has never been seen before. When the preQ1 molecule ties, the riboswitch twists to communicate to the RNAP for the transcription to be continued.
Further, these observations were rationalized and validated via a collaborative effort with Frank, then a Professor of biophysics and chemistry at the University of Michigan and an expert in the computational modeling of RNAs.
With the availability of elaborate 3D models in hand, the U-M collaborative group currently has a highly precise knowledge of how this riboswitch controls transcriptional pausing.
Now we understand the whole process of riboswitch regulation and can use that knowledge to specifically target these critical parts of bacterial life, hopefully averting the coming crisis of multidrug-resistant bacteria.”
Nils Walter, Professor, Chemistry, University of Michigan
Source:
Journal reference:
Chauvier, A., et al. (2023). Structural basis for control of bacterial RNA polymerase pausing by a riboswitch and its ligand. Nature Structural & Molecular Biology. doi.org/10.1038/s41594-023-01002-x.