According to a study from scientists at Weill Cornell Medicine and the National Heart, Lung, and Blood Institute, a division of the National Institutes of Health, DNA can imitate protein activities by folding into complex, three-dimensional structures.
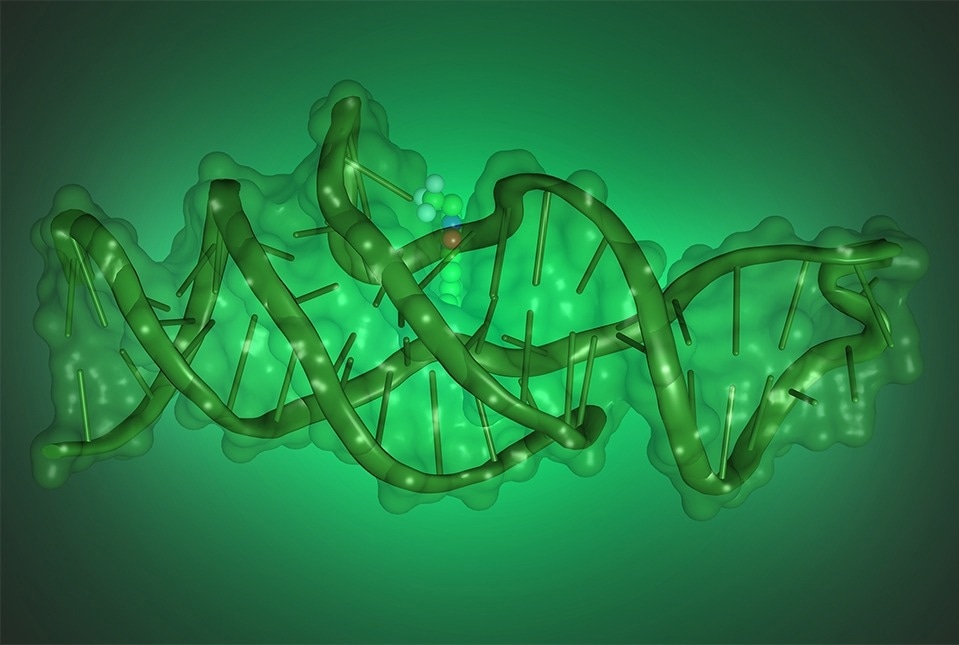 Illustration showing the structure of Lettuce, a DNA that binds and activates fluorophores derived from green fluorescent protein. Image Credit: Luiz F.M. Passalacqua.
Illustration showing the structure of Lettuce, a DNA that binds and activates fluorophores derived from green fluorescent protein. Image Credit: Luiz F.M. Passalacqua.
The DNA molecule they constructed to replicate the function of a protein called green fluorescent protein (GFP) was shown to have a novel and complicated structure by the researchers in their study, which was published on June 21st, 2023, in Nature.
GFP, a jellyfish-derived protein that serves as a fluorescent tag or beacon in cells, has developed into a crucial scientific tool.
The results contribute to the understanding of how DNA can be engineered to fold into complex shapes and will aid in the development of such DNA molecules for a range of laboratory and clinical uses.
For example, an all-DNA fluorescent tag that mimics GFP would frequently be excellent for marking specific DNA segments in biological studies and diagnostic test kits and would be reasonably easy to produce.
These findings really change our understanding of what we can do with DNA.”
Dr. Samie Jaffrey, Greenberg-Starr Professor, Pharmacology, Weill Cornell Medicine
Most DNA in nature is found as a double-stranded, “twisted ladder” or “helical” structure that functions as a relatively stable genetic data storage. Other molecules, particularly proteins, carry out all of the other intricate biological operations in cells.
One such molecule was identified by Dr. Jaffrey and colleagues last year. It is a single-stranded DNA that has been folded in a manner that allows it to mimic the activity of GFP. Dr. Jaffrey gave the DNA molecule the nickname “lettuce” due to the color of its fluorescent emissions.
The DNA molecule functions by attaching to a different small organic molecule, a potentially fluorescent “fluorophore” like the one at the center of GFP, and squeezing it in a way that activates its ability to fluoresce. The researchers proved the combination of lettuce and fluorophore as a fluorescent tag for the quick detection of COVID-19’s causative agent, SARS-CoV-2.
Making a large number of single-stranded DNAs and screening them for those that have the requisite fluorophore-activating properties allowed Dr. Jaffrey and his colleagues to discover lettuce.
However, they were unsure of what mechanism lettuce had employed to get this ability. They consulted their longtime partner and NHLBI Senior Investigator Dr. Adrian R. Ferré-D’Amaré in the new study to discover that structure.
The structure of lettuce was resolved at the atomic level in the study conducted by Dr. Luiz Passalacqua, a research fellow on Dr. Ferré-D’Amaré’s team, using advanced structural imaging techniques, such as cryo-electron microscopy.
They discovered that it folds into a form with an unusual four-way DNA junction at its core, enclosing the fluorophore and activating it. They also noticed that the nucleobase bonds, frequently referred to as the “letters” in the four-letter DNA alphabet, hold the folding of lettuce together.
What we have discovered is not DNA trying to be like a protein; it is a DNA that is doing what GFP does but in its own special way.”
Dr. Adrian R. Ferré-D’Amaré, Senior Investigator, National Heart, Lung, and Blood Institute
The findings, according to the researchers, should hasten the production of fluorescent DNA molecules like lettuce for quick diagnostic tests and a variety of other scientific applications where a DNA-based fluorescent tag is needed.
Dr. Jaffrey concluded, “Studies like this are going to be essential for the creation of new DNA-based tools.”
Source:
Journal references:
Passalacqua, L. F. M., et al. (2023). Intricate 3D architecture of a DNA mimic of GFP. Nature. doi.org/10.1038/s41586-023-06229-8
VarnBuhler, B, S., et al. (2023). Detection of SARS-CoV-2 RNA Using a DNA Aptamer Mimic of Green Fluorescent Protein. Nature. doi.org/10.1021/acschembio.1c00893