Reviewed by Danielle Ellis, B.Sc.Jun 26 2023
It can be extremely helpful to learn living cells’ intricate structure and assembly using real-world models that resemble them. These models can be created through synthetic techniques, but they are costly, time-consuming, and experimentally difficult. Microdroplets that entrap biological materials are a prototype of these models.
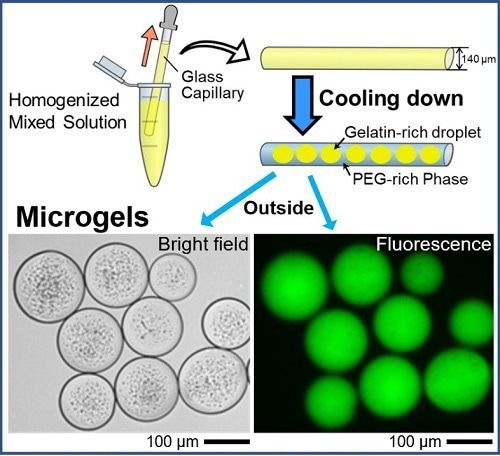 Spontaneous formation of uniform cell-sized microgels inside a glass capillary is reported. By adapting aqueous polymer solution containing DNA, droplets entrapping DNA are generated in a self-organized manner through micro phase-separation, and these droplets are transformed into gel state by decreasing the temperature. The microgels are easily extruded into bulk water, maintaining their size. Image Credit: Akihisa Shioi from Doshisha University
Spontaneous formation of uniform cell-sized microgels inside a glass capillary is reported. By adapting aqueous polymer solution containing DNA, droplets entrapping DNA are generated in a self-organized manner through micro phase-separation, and these droplets are transformed into gel state by decreasing the temperature. The microgels are easily extruded into bulk water, maintaining their size. Image Credit: Akihisa Shioi from Doshisha University
The living cell contains proteins and genetic material (DNA) that are relevant to physiology in a “self-organized” environment. Understanding this self-assembly process can help better comprehend how living things organize themselves.
To study cellular self-assembly, water/oil (w/o) or water/water (w/w) droplets can be employed as prototypes or “models” that resemble cells. Significant implications for the realm of biomedical research are also provided by these models.
Although cell mimetics can be produced using complex, expensive technology, the associated processes are expensive, time-consuming, and difficult.

Image Credit: Andrii Vodolazhskyi/Shutterstock.com
Recently, Japanese scientists were able to create a one-step procedure for creating homogenous gelatin-based cell mimetics known as “microgels.” On May 24th, 2023, the associated findings were published in the journal Small.
Mayu Shono and Prof. Akihisa Shioi from Doshisha University, who led the study, added, “Currently, our research focuses on understanding the self-organization of living matter. As an extension of our research activity, we have discovered an experimental procedure that may be quite useful for the generation of microgels.”
Gen Honda and Miho Yanagisawa from The University of Tokyo, as well as Kenichi Yoshikawa from Doshisha University and Kyoto University, were also members of the research team.
It is fascinating to learn how microgels are created. The first step entails the creation of domain structures made of gelatin and polyethylene glycol (PEG), two popular synthetic crosslinkers. The gelatin-rich domain easily enters the gel phase when the temperature is lowered to 24 °C.
Due to its stronger affinity for glass and weaker affinity for the gelatin-rich domains, the PEG-rich phase migrates preferentially to the glass surface of the capillary tube under a specific set of experimental conditions. As a result, the PEG-rich phase absorbs the gelatin-rich droplets.
Glass capillary tests were also used to validate these results in theoretical and numerical modeling investigations, which supported the notion that w/w phase separation was primarily driven by the inner glass capillary’s wettability.
The phase separation of PEG and gelatin also allowed the gelatin-rich droplets to spontaneously entrap DNA molecules after DNA was added, resulting in the formation of microgels that mimic cells.
The study found that even above the sol/gel transition temperature, the negatively charged DNA molecules included in the droplets could keep them stable by preventing their fusion.
The team also labeled and monitored the DNA that was encapsulated using a fluorescent dye. Later fluorescent microscopy tests identified circular microgel formations containing the glowing DNA molecules. The authors predict that the current method will contain, store, and transport massive DNA molecules inside microscopic cell-sized droplets.
This novel method to form uniform cell-sized microgels may be applicable to other biopolymers. The uniform cell-sized and stable cell-like systems will also have key implications in the area of biological and life sciences.”
Mayu Shono, Study First Author and PhD Student, Department of Chemical Engineering and Materials Science, Doshisha University
In conclusion, the study provides a novel approach for the production of gelatin-based cell mimics that could be tailored to the specific application to achieve the required results.
Prof. Shioi concluded, “The method proposed in our study, which does not require special equipment, organic solvents, or surfactants, may be useful for producing microgels for food, medicines, cosmetics, and other materials.”
Source:
Journal reference:
Shono, M., et al. (2023). Spontaneous Formation of Uniform Cell-Sized Microgels through Water/Water Phase Separation. Small. doi.org/10.1002/smll.202302193