A recent study supported by Tokyo Tech’s World Research Hub Initiative discovered that gene expression within the apicoplast, an organelle in the malaria parasite Plasmodium falciparum, is controlled by melatonin (the circadian signaling hormone) in host blood and intrinsic parasite cues via a factor called ApSigma. This study’s emphasized regulatory mechanism could be a future target for malaria treatment.
 Gene expression within the apicoplast, an organelle in the malaria parasite Plasmodium falciparum, is regulated by melatonin (the circadian signaling hormone) in host blood, and intrinsic parasite cues, via a factor called ApSigma, as identified by a recent study aided by Tokyo Tech’s World Research Hub Initiative. The regulatory system highlighted in this study might be a future target for malaria treatment. Image Credit: Professor Kan Tanaka, Tokyo Institute of Technology
Gene expression within the apicoplast, an organelle in the malaria parasite Plasmodium falciparum, is regulated by melatonin (the circadian signaling hormone) in host blood, and intrinsic parasite cues, via a factor called ApSigma, as identified by a recent study aided by Tokyo Tech’s World Research Hub Initiative. The regulatory system highlighted in this study might be a future target for malaria treatment. Image Credit: Professor Kan Tanaka, Tokyo Institute of Technology
With 240 million people worldwide suffering from malaria each year, it is one of the major public health risks. This fatal condition is not communicable, though. It is spread through the bite of a female Anopheles mosquito carrying the Plasmodium falciparum malaria parasite.
This parasite enters the body of humans through mosquito bites and produces extremely recurrent symptoms such as fever, cold, weariness, and headache. The circadian rhythm, or the host’s 24-hour internal biological clock, and the parasite's life cycle can be linked as the cause of the symptoms’ periodicity.
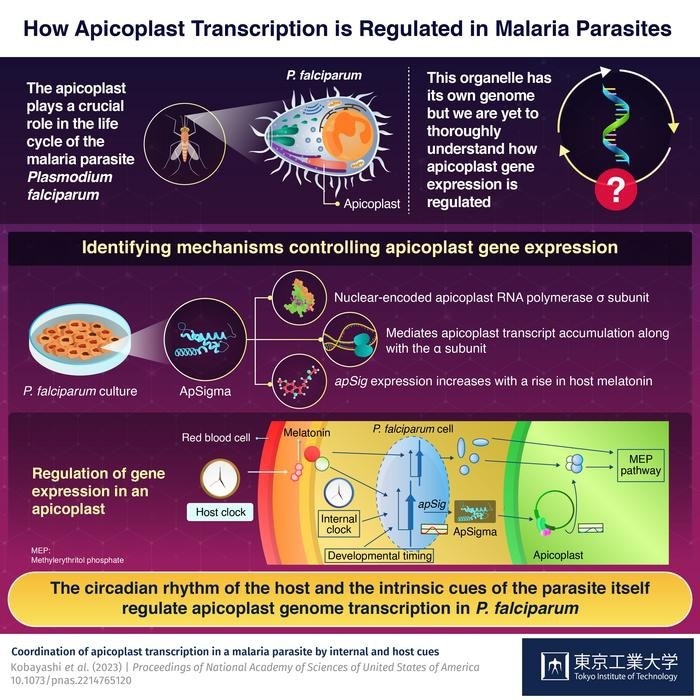
P. falciparum contains an apicoplast, a unique cellular organelle that contains its genome and is crucial for the parasite’s life cycle. Despite its importance, however, not much is known about the mechanisms regulating gene expression in apicoplasts and their potential role in modulating the observed periodicity of malaria symptoms, or the life cycle of P. falciparum.
For this reason, a group of researchers from the Tokyo Institute of Technology (Tokyo Tech), under the direction of Professor Kan Tanaka recently launched a cooperative investigation to better understand the fundamental mechanisms that regulate apicoplast gene expression.
The collaboration with co-authors Professor Kiyoshi Kita of Nagasaki University and Professor Antony N. Dodd, a group leader at the John Innes Centre in the UK and a visiting professor at Tokyo Tech, was made possible by the institute’s World Research Hub Initiative (WRHI), a project for interdisciplinary collaboration with top researchers from around the world.
The study was published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
Previous studies have shown that certain plant σ subunits participate in the circadian regulation of gene expression in plastids (i.e., organelles like the apicoplast). Therefore, the present study hypothesized that a nuclear-encoded σ subunit might coordinate apicoplast gene expression with the life cycle of P. falciparum or the circadian rhythm of its host.”
Kan Tanaka, Professor, Tokyo Institute of Technology
The team cultivated P. falciparum in a lab and used immunofluorescence microscopy and phylogenetic analysis to study it. As a result, they discovered ApSigma, a nuclear-encoded σ subunit of the apicoplast RNA polymerase.
It most likely mediates apicoplast transcript accumulation, which has a periodicity similar to that of the parasite’s developmental control, together with the subunit. Melatonin, the circadian signaling hormone found in host blood, also caused an increase in apicoplast transcription and expression of the apicoplast subunit gene, apSig.
The researchers propose that there is an evolutionarily maintained regulatory system in which the host’s circadian rhythm is merged with the parasite’s intrinsic cues based on the data gathered from various studies.
They work together to orchestrate genome transcription in P. falciparum apicoplast. This study sets a strong foundation for future research in the field that aims to fully explain the regulatory mechanisms of the Plasmodium cell cycle.
Prof. Tanaka concludes by highlighting the ramifications of the current findings for the future.
He concluded, “Malaria kills hundreds of thousands of people across the world, every year. This study identifies a regulatory system that might be a future target for malaria treatment.”
Professor Dodd, added, “It is amazing that a process we identified in plants has led to the discovery of an equivalent mechanism in a globally important pathogen. The new protein and mechanism identified could present a new target for the development of drugs for the treatment and or prevention of malaria, in both humans and farm animals.”
Professor Kita concluded, “This research demonstrates the value of international and interdisciplinary collaboration, and the power of plant sciences and microbiology to drive unusual and novel discoveries that could be of considerable global benefit.”