N6-methyladenosine (m6A) is the most extensively studied RNA modification across multiple species, and its importance in the immune system has been demonstrated in a variety of contexts, including mRNA metabolism, cell differentiation, proliferation, and response to stimulation.
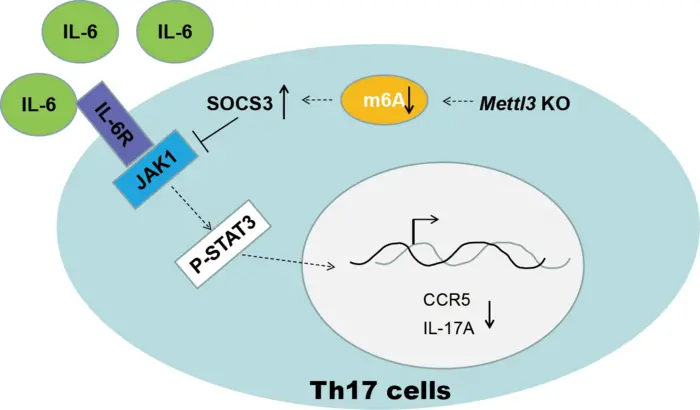 Loss of METTL3 in Th17 cells facilitated SOCS family RNA stability, inhibited IL-6/STAT3 mediated IL-17A and CCR5 expression, which in turn impeding Th17 cells differentiation and infiltration, eventually attenuating the process of EAE. Image Credit: ©Science China Press
Loss of METTL3 in Th17 cells facilitated SOCS family RNA stability, inhibited IL-6/STAT3 mediated IL-17A and CCR5 expression, which in turn impeding Th17 cells differentiation and infiltration, eventually attenuating the process of EAE. Image Credit: ©Science China Press
Previous research from the Hua-Bing Li group showed that the m6A methyltransferase METTL3 regulates T cell homeostasis and maintains the suppressive function of regulatory T cells (Tregs). However, the function of m6A methyltransferase in other T cell subtypes is unknown.
T helper cells 17 (Th17) are important in both host defense and autoimmunity. The researchers discovered that METTL3 loss in T cells caused a major defect in Th17 cell differentiation and slowed the development of experimental autoimmune encephalomyelitis (EAE).
Image Credit: Design_Cells/Shutterstock.com
They created Mettl3f/fIl17aCre mice and found that METTL3 deficiency in Th17 cells substantially suppressed the development of EAE and showcased less Th17 cells infiltration into CNS.
The m6A modification has been linked to RNA metabolism, specifically affecting RNA stability. SOCS gene mRNAs have been identified as m6A targets in CD4+ T cells, and deletion of METTL3 reduced SOCS mRNA decay.
Researchers confirmed that METTL3 depletion enabled SOCS3 RNA stability, which then reduced IL-17A and CCR5 expression, disrupted Th17 cell differentiation and infiltration, and eventually reduced the process of EAE.
The researchers conclude that m6A modification preserves Th17 cell function, providing new insights into the regulatory network of Th17 cells and implying a potential therapeutic target for Th17 cell-mediated autoimmune disease.
Source:
Journal reference:
Wang, X., et al. (2023). m6A mRNA modification potentiates Th17 functions to inflame autoimmunity. Science China Life Sciences. doi.org/10.1007/s11427-022-2323-4.