Reviewed by Danielle Ellis, B.Sc.Aug 2 2023
A group of researchers guided by the University of Oxford has made significant progress in detecting changes in protein structures. The method, which was published in Nature Nanotechnology, uses novel nanopore technology to detect structural variations at the single-molecule level, even deep within long protein chains.
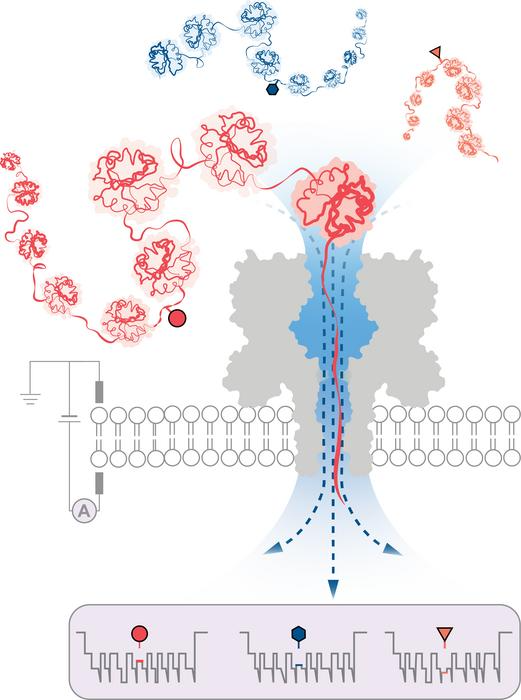 An engineered protein nanopore directed a water flux strong enough to capture, unfold and translocate proteins exceeding 1200 amino acids in length. Modulation of electrical current during protein translocation through the nanopore detected post-translational modifications deep within the proteins (shown as circle, triangle, and hexagon). Image Credit: Wei-Hsuan Lan, and Yujia Qing.
An engineered protein nanopore directed a water flux strong enough to capture, unfold and translocate proteins exceeding 1200 amino acids in length. Modulation of electrical current during protein translocation through the nanopore detected post-translational modifications deep within the proteins (shown as circle, triangle, and hexagon). Image Credit: Wei-Hsuan Lan, and Yujia Qing.
There are approximately 20,000 protein-encoding genes in human cells. The actual number of proteins observed in cells, however, is much higher, with over 1,000,000 different structures known.
These variants are produced by a process known as post-translational modification (PTM), which takes place after a protein has been transcribed from DNA. PTM introduces structural changes to individual amino acids that make up proteins, like the addition of chemical groups or carbohydrate chains. As a result, the same protein chain can have hundreds of different variations.
Image Credit: nobeastsofierce/Shutterstock.com
These variants are important in biology because they allow for the precise regulation of complex biological processes within individual cells. Mapping this variation would reveal a wealth of useful information that could transform the knowledge of cellular functions. However, the ability to generate comprehensive protein inventories has remained elusive to date.
To address this, a group led by researchers from the Department of Chemistry at the University of Oxford efficaciously devised a technique for protein analysis based on nanopore DNA/RNA sequencing technology.
In this method, a directional flow of water captures and unfolds 3D proteins into linear chains that are loaded through tiny pores just wide enough for a single amino acid molecule to pass through. The structural variations are distinguished by measuring changes in an electrical current applied across the nanopore. Different molecules disrupt the current in different ways, giving them a distinct signature.
The researchers successfully demonstrated the method’s ability to detect three different PTM modifications (phosphorylation, glutathionylation, and glycosylation) at the single-molecule level for protein chains longer than 1,200 residues. These included changes that were made deep within the protein’s sequence. Notably, no labels, enzymes, or additional reagents are required for the method.
The investigators stated that the new protein characterization method could be easily incorporated into portable nanopore sequencing devices already in use, allowing for the quick formation of protein inventories of individual cells and tissues. By enabling the personalized detection of particular protein variants linked to diseases like cancer and neurodegenerative disorders, this may enable point-of-care diagnostics.
This simple yet powerful method opens up numerous possibilities. Initially, it allows for the examination of individual proteins, such as those involved in specific diseases. In the longer term, the method holds the potential to create extended inventories of protein variants within cells, unlocking deeper insights into cellular processes and disease mechanisms.”
Yujia Qing, Professor, Department of Chemistry, University of Oxford
“The ability to pinpoint and identify post-translational modifications and other protein variations at the single-molecule level holds immense promise for advancing our understanding of cellular functions and molecular interactions. It may also open new avenues for personalized medicine, diagnostics, and therapeutic interventions,” states Professor Hagan Bayley (Department of Chemistry, University of Oxford), contributing author and co-founder of Oxford Nanopore Technologies.
Source:
Journal reference:
Martin-Baniandres, P., et al. (2023). Enzyme-less nanopore detection of post-translational modifications within long polypeptides. Nature Nanotechnology. doi.org/10.1038/s41565-023-01462-8.