From impacting how the human body stores fat to how the brain regulates appetite, hundreds of genes and environmental factors jointly identify weight and body size.
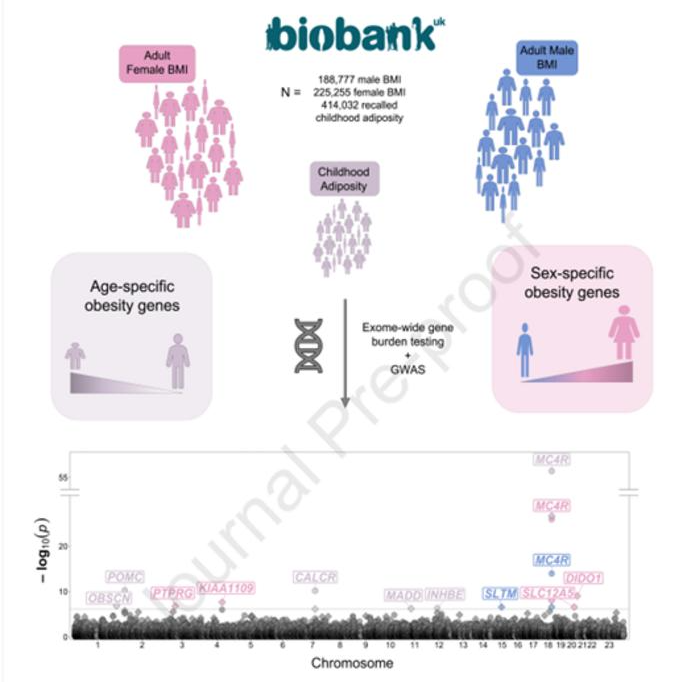 Researchers identified age-specific and sex-specific obesity genes by looking into the genome of 414,032 people from the UK. Image Credit: Cell Genomics/Kaisinger et al.
Researchers identified age-specific and sex-specific obesity genes by looking into the genome of 414,032 people from the UK. Image Credit: Cell Genomics/Kaisinger et al.
The study, which was published in Cell Genomics, might offer insights into new biological pathways that underlie obesity and stress how sex and age add up to health and disease.
There are a million and one reasons why we should be thinking about sex, age, and other specific mechanisms rather than just lumping everyone together and assuming that disease mechanism works the same way for everyone. We’re not expecting people to have completely different biology, but you can imagine things like hormones and physiology can contribute to specific risks.
John Perry, Study Senior Author, Geneticist and Professor, Wellcome-MRC Institute of Metabolic Science, University of Cambridge
To unravel the role of sex in obesity risk, the research group sequenced the exome—the protein-coding part of the genome—of 414,032 adults from the UK Biobank study.
They considered variants, or mutations, within genes linked to the body mass index (BMI) in men and women. Depending on height and weight, BMI is an estimated measurement of obesity. The search turned up five genes impacting BMI in women and two in men.
Amongst them, faulty variants of three genes—DIDO1, PTPRG, and SLC12A5—are associated with higher BMI in women, up to almost 8 kg/m² more, while having no impact on men. More than 80% of the women with SLC12A5 and DIDO1 variants had obesity, as around by their BMI.
Individuals carrying DIDO1 variants had stronger links with greater testosterone levels and increased waist-to-hip ratio, both considered risk indicators for obesity-related complications like heart disease and diabetes.
Compared with non-carriers, others with SLC12A5 variants had greater odds of having type 2 diabetes. Such outcomes stress earlier undiscovered genes implicated in the development of obesity in women but not men.
Furthermore, Perry and his colleague repeated their technique to look for age-specific factors by looking for gene variants linked to childhood body size depending on participants’ recollections.
Two genes, named OBSCN and MADD, were found by the team that were not earlier associated with childhood body size and fat. While carriers of OBSCN variants consisted of higher odds of having higher weight as a child, MADD variant carriers were linked to smaller body sizes. Besides, the genetic variants acting on MADD had no link with adult obesity risk, thereby emphasizing the age-specific effects on body size.
What’s quite surprising is that if you look at the function of some of these genes that we identified, several are clearly involved in DNA damage response and cell death. There’s currently no well-understood biological paradigm for how DNA damage response would influence body size. These findings have given us a signpost to suggest variation in this important biological process may play a role in the etiology of obesity.
John Perry, Study Senior Author, Geneticist, and Professor, Wellcome-MRC Institute of Metabolic Science, University of Cambridge
Obesity is known as a brain-related disorder, whereas environmental and biological factors act to impact appetite.
Next, the research group believes to repeat the study in a bigger and highly diverse population. Also, they plan to study the genes in animals to explore their function and also the relationship with obesity.
We’re at the very earliest stages of identifying interesting biology. We hope the study can reveal new biological pathways that may one day pave the way to new drug discovery for obesity.
John Perry, Study Senior Author, Geneticist, and Professor, Wellcome-MRC Institute of Metabolic Science, University of Cambridge
Source:
Journal reference:
Kaisinger, L. R., et al. (2023) Large-scale exome sequence analysis identifies sex- and age-specific determinants of obesity. Cell Genomics. doi.org/10.1016/j.xgen.2023.100362.