Different cells in the human pancreas play important roles in blood sugar regulation. Neurogenin 3 (NEUROG3) is a gene found in pancreatic cells. Diabetes can result from its mutation. It is only active for a short period of time during pancreas development, so its behavior and dynamics have remained a mystery, particularly in the context of human development.
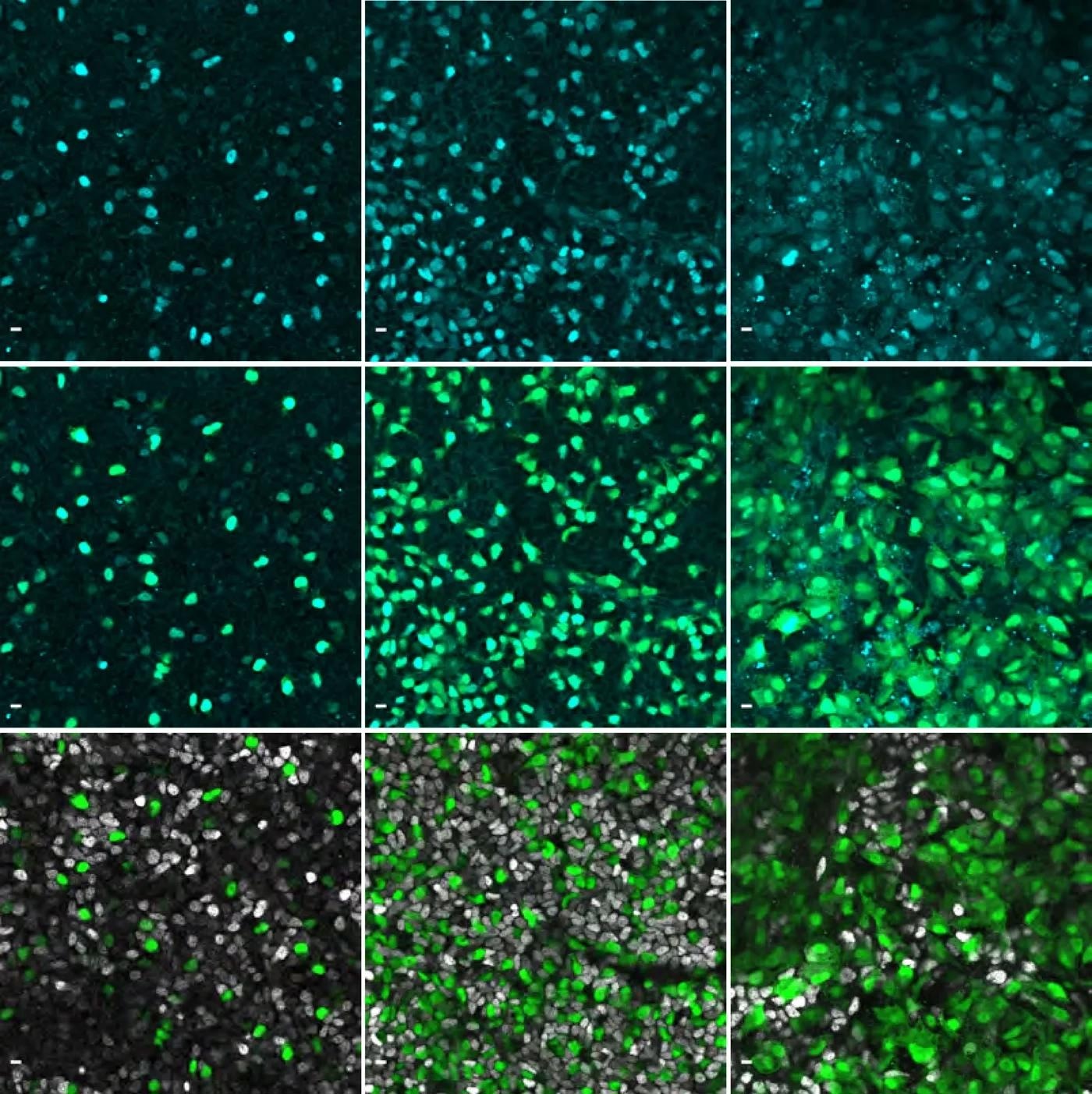 Immunofluorescence images showing the gene NEUROG3 in cyan in human pancreatic cells. Image Credit: © Beydag-Tasöz et al., Developmental Cell, MPI-CBG
Immunofluorescence images showing the gene NEUROG3 in cyan in human pancreatic cells. Image Credit: © Beydag-Tasöz et al., Developmental Cell, MPI-CBG
To fully understand the gene, scientists from the Max Planck Institute of Molecular Cell Biology and Genetics in Dresden, Germany, and the Novo Nordisk Foundation at the University of Copenhagen, Denmark, used a unique method to monitor both the gene’s activity and the protein it produces in human pancreas cells.
The investigators created a method for linking the dynamic behaviors of pancreatic cells seen in live imaging movies to all the genes they express. This will contribute to a deeper understanding of how the pancreas’ hormone-producing cells develop and may lay the groundwork for more of these cells to be produced for therapeutic purposes, like the production and transplantation of these cells to diabetic patients.
The pancreas’s various cells, like beta cells that produce insulin, regulate human blood sugar. Insulin aids in the regulation of blood sugar levels. Diabetes can develop if these cells stop working or die. When human bodies are developing, all of these special cells arise from a single cell type in the pancreas known as a pancreatic endocrine progenitor. For a brief period of time, this cell relies on a gene called NEUROG3 to perform its function.
The research team led by Anne Grapin-Botton, Managing Director of the Max Planck Institute of Molecular Cell Biology and Genetics, and coworkers from the Novo Nordisk Foundation at the University of Copenhagen wanted to discover more about these special cells in the pancreas that use the gene NEUROG3, as well as to investigate how this gene behaves in single cells.
Different Gene Activity in Different Cells
“We used special tags to see NEUROG3 in these cells and watched how they move using a long-term live imaging method that generated videos,” explains Belin Selcen Beydag-Tasöz, the first author of the study.
By looking at flat 2D and 3D models of human pancreas growth, we found out that the levels of the NEUROG3 gene were different in different cells. Some cells had a lot of this gene, and some had a little. Surprisingly, despite this heterogeneity, all the cells that had detectable NEUROG3 formed cells producing hormones. Another surprising result was that NEUROG3 works about two times slower in humans than in mice, meaning it takes more time for the gene to do its job in humans compared to mice.”
Belin Selcen Beydag-Tasöz, Study First Author, Max Planck Institute of Molecular Cell Biology and Genetics
The investigators utilized long-term live imaging to observe a process normally hidden within the mother’s womb. The brightness of the cells aided them in combining gene activity with cell behavior. Using this method, the researchers discovered that when the NEUROG3 gene becomes active, another gene called KLK12 causes cells to move and begin forming islets of Langerhans.
The cell culture systems we developed to understand how cells in human fetuses form organs are starting to bear fruit. In our study, we've learned a lot more about how certain genes activity during fetal development can lead to diabetes later in life. The results show that when producing endocrine cells for future therapeutic applications based on the transplantation of these cells into diabetic patients, there is some flexibility in the control of NEUROG3.”
Anne Grapin-Botton, Max Planck Institute of Molecular Cell Biology and Genetics
Source:
Journal reference:
Beydag-Tasöz, B. S., et al. (2023). Integrating single-cell imaging and RNA sequencing datasets links differentiation and morphogenetic dynamics of human pancreatic endocrine progenitors. Developmental Cell. doi.org/10.1016/j.devcel.2023.07.019.