One of immunology’s unsolved mysteries is the precise mechanism by which B cells make millions of antibodies with varied specificities to protect us against a variety of diseases.
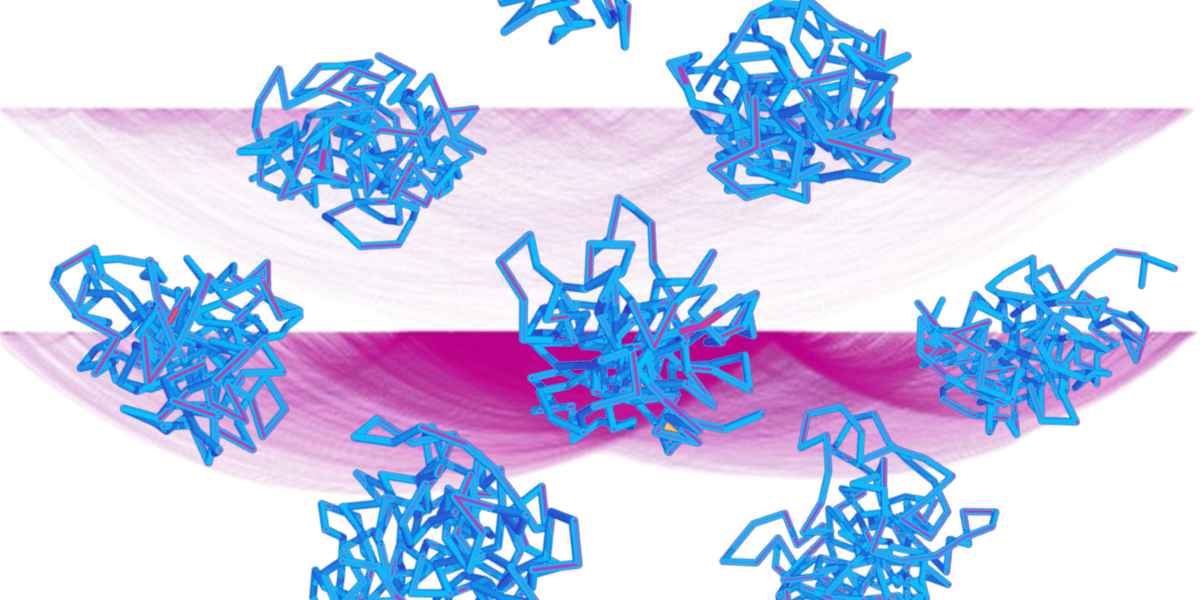 The image shows a visual representation of the research, utilizing and adapting figures from the research publication. The two background shapes illustrate chromosomal interaction mapping for two types of related techniques, showing an enrichment in the detection of chromosomal DNA interactions for the method used in this research for pro-B cells (lower shape) taken from figure 1C. The foreground shapes show the variation in DNA conformation at the immunoglobulin heavy chain locus from different pro-B cells. Image Credit: Babraham Institute.
The image shows a visual representation of the research, utilizing and adapting figures from the research publication. The two background shapes illustrate chromosomal interaction mapping for two types of related techniques, showing an enrichment in the detection of chromosomal DNA interactions for the method used in this research for pro-B cells (lower shape) taken from figure 1C. The foreground shapes show the variation in DNA conformation at the immunoglobulin heavy chain locus from different pro-B cells. Image Credit: Babraham Institute.
The Babraham Institute researchers have demonstrated how specific DNA connections in each B cell are at the core of antibody variation by utilizing their expertise in 3D genome organization and antibody variation studies. With more research, their findings could shed light on why antibody diversity drops with age and offer therapies to promote health in later life.
Researchers from a variety of fields, including immunologists, bioinformaticians, biophysicists, and pioneers in 3D genome research, under the direction of Dr. Anne Corcoran at the Babraham Institute, have revealed that the 3D organization of DNA in mouse B cells permits physically distant genes to join together during antibody creation and generate the variety of antibodies required for effective disease defense.
Unexpectedly, they discovered that every B cell folds this portion of the genome differently rather than displaying any consistent folding, indicating that there are countless ways to arrange the genes in a certain order.
We wanted to understand the mechanisms behind antibody variety. One way the cell achieves this is cutting and pasting from a suite of options for the antibody genes, but the puzzling thing is genes that are far away from the location of this event are used just as often as ones close by, so there must be some way of bringing everything together and making sure that everything needed is at hand.
Dr. Anne Corcoran, The Babraham Institute
Dr. Anne Corcoran is the Senior group leader of the Immunology program.
Previously, researchers faced limitations in examining these mechanisms, leaving two divergent perspectives. One school of thought posited that DNA’s arrangement would exhibit flexibility, while the other hypothesized the presence of shared principles governing the folding process.
However, thanks to the Corcoran lab’s sophisticated methodology, they have now achieved a breakthrough by mapping chromosome interactions in high resolution for the first time, conclusively settling the debate.
The team created a method for next-generation sequencing that enabled them to collect more detailed data on DNA configuration from B cells. The method was derived using the Institute’s enriched Hi-C genome analysis technology, which locates direct chromosomal contacts across the genome at high resolution.
They were able to learn more about the interactions between chromosomes and DNA looping, thanks to this enriched data set. The Friedrich Miescher Institute (FMI) in Basel, Switzerland, and the Babraham team worked together to create 3D simulations of thousands of gene structures to support their findings.
In addition to mapping the interactions among the antibody genes, the research team also identified connections between crucial genes that facilitate the maturation of B cells in a manner specific to their developmental stage and cell type. The team suggests that these chromosomal contacts likely play a vital role in ensuring the accurate and synchronized regulation of gene expression during the B cell’s maturation process.
We found that the antibody genes were in close proximity to a small proportion of other genes on different chromosomes. Once we characterized them we found that these genes were associated with B cell development, and that was very interesting to see because if we apply this approach to other cell types we might be able to find important chromosomal interactions that are unique to those cell types.
Dr. Anne Corcoran, The Babraham Institute
The team proposes that the arrangement of DNA may exert an influence on the number of distinct antibodies generated by the body as it undergoes aging. With age, human bodies tend to produce a narrower spectrum of antibodies because B cells choose fewer genes from the extensive repertoire available.
Notably, they tend to utilize fewer genes situated further away in the genome. This phenomenon could be attributed to alterations in the DNA folding mechanism, potentially rendering certain genes “inaccessible” and no longer favored for antibody production. The next phase of this research will involve an exploration of the 3D organization of genes responsible for specifying antibodies within aging B cells.
Source:
Journal reference:
Mielczarek, O., et al. (2023). Intra- and interchromosomal contact mapping reveals the Igh locus has extensive conformational heterogeneity and interacts with B-lineage genes. Cell Reports. doi.org/10.1016/j.celrep.2023.113074.