Reviewed by Danielle Ellis, B.Sc.Sep 12 2023
A fascinating phenomenon of cellular reprogramming in developed adult organs has been revealed by researchers, providing insight into a brand-new process of adaptive development. The research, which was done on fruit flies (Drosophila), sheds further light on the process of dedifferentiation, in which cells with defined roles become less specialized, undifferentiated cells, such as stem cells.
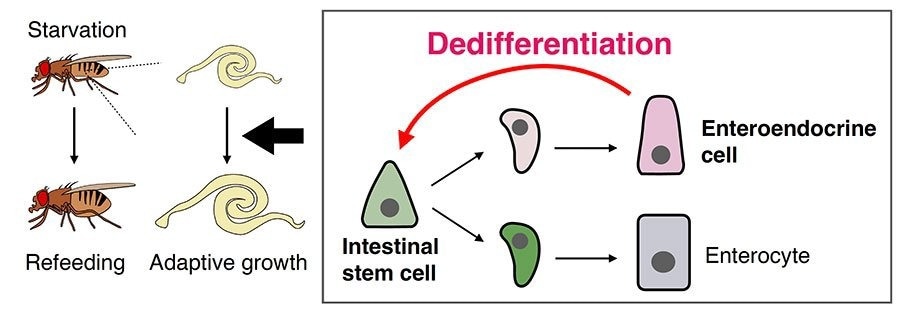 A brief summary of this study: the Drosophila adult midgut rapidly grows in size upon recovery from starvation (adaptive growth). Image Credit: Hiroki Nagai et al.
A brief summary of this study: the Drosophila adult midgut rapidly grows in size upon recovery from starvation (adaptive growth). Image Credit: Hiroki Nagai et al.
Up until recently, dedifferentiation has mostly been linked to serious injuries or demanding situations, seen in diseases like cancer and tissue regeneration. Enteroendocrine cells (EEs) inside the intestinal epithelium undergo dedifferentiation into intestinal stem cells (ISCs) in response to nutritional changes, such as recovery from fasting, according to a new discovery made by the researchers.
Through meticulous experimentation, we identified a subset of enteroendocrine cells residing in the adult midgut of Drosophila, which exhibit dedifferentiation into ISCs when nutrient levels fluctuate. By utilizing in vivo lineage tracing of EEs and single-cell RNA sequencing, we pinpointed the dedifferentiating EE subpopulation and developed a genetic system for selectively removing ISCs derived from dedifferentiation, a process known as ablation.”
Hiroki Nagai, Study First Author and Postdoctoral Researcher, Tohoku University
Surprisingly, the ablation tests showed that dedifferentiation is essential for ISC proliferation and subsequent intestine growth after ingesting food. Massive stem cell ablation was used in earlier research using mice to cause dedifferentiation. However, in the current study, stem cells did not decrease but rather proliferated in response to dietary cues.
This key difference shows that dedifferentiation contributes considerably to organ remodeling throughout environmental adaptations and is not just restricted to regenerative situations.
Image Credit: Yurchanka Siarhei/Shutterstock.com
The researchers also uncovered the molecular mechanism behind nutrient-dependent dedifferentiation, which involves EEs activating the JAK-STAT signaling pathway in response to dietary deficiencies in amino acids and glucose, which facilitates the transformation of EEs into ISCs during post-starvation recovery.
This suggests that the nutrient-dependent dedifferentiation could be an evolutionary conserved process across species when paired with results from previous investigations.
This might enable artificial cellular reprogramming to be managed in vivo, according to Yuichiro Nakajima, who was formerly located at FRIS and is the study’s corresponding author.
If we figure out specific nutrients and the detailed signaling that induce dedifferentiation, we could control cell fate plasticity by nutritional intervention and/or pharmacological treatments.”
Yuichiro Nakajima, Study Corresponding Author and Lecturer, Graduate School of Pharmaceutical Sciences, University of Tokyo
In the future, they aim to study cell fate plasticity under physiological factors other than nutrition, such as reproduction, temperature, light, and exercise. This might lead to the discovery of new processes underpinning environmental adaptations.
Source:
Journal reference:
Nagai, H., et al. (2023). Nutrient-driven dedifferentiation of enteroendocrine cells promotes adaptive intestinal growth in Drosophila. Developmental Cell. doi.org/10.1016/j.devcel.2023.08.022