Reviewed by Danielle Ellis, B.Sc.Sep 19 2023
The development of technology that makes protein sequencing simple has been the focus of an actual race among scientists. Giovanni Maglia, a professor of chemical biology at the University of Groningen, has now discovered the final component of the puzzle: a method for moving a protein through a nanopore, enabling the sequencing of proteins with a simple, portable device.
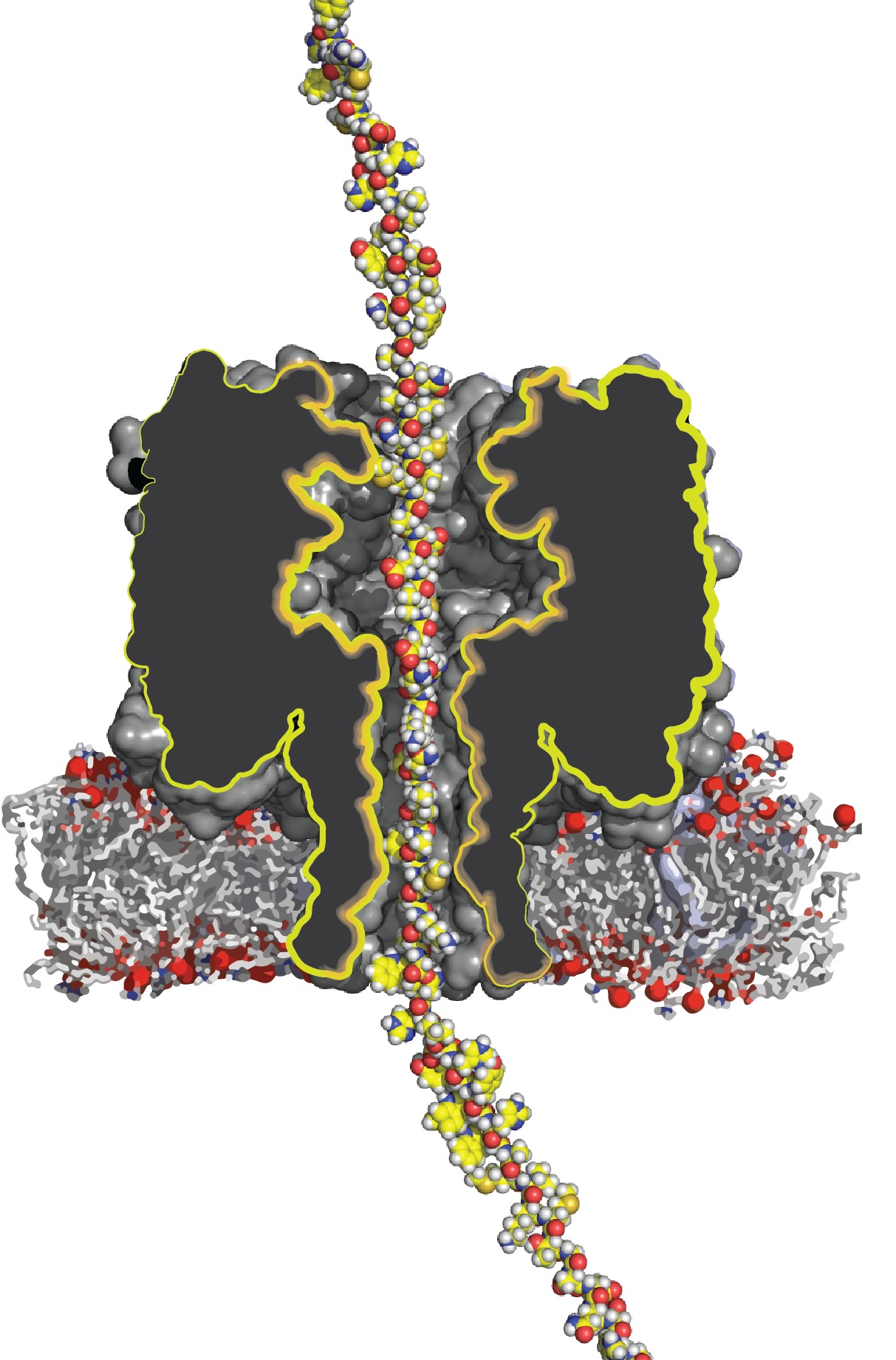 Artist impression of a protein transported through a nanopore. Image Credit: Giovanni Maglia
Artist impression of a protein transported through a nanopore. Image Credit: Giovanni Maglia
Sequencing proteins is the next great challenge after DNA sequencing, which has revolutionized the comprehension of life.
DNA is mostly static. The processes in our cells are executed by proteins: they do the actual work. And by understanding proteins, we will understand even more about how our bodies work.”
Giovanni Maglia, Professor, Chemical Biology, University of Groninger
The Problem of Pulling Proteins Through a Hole
There are presently portable DNA sequencing instruments on the market. These devices employ nanopore technology, in which a single strand of DNA is dragged through a small hole (a nanopore) in a membrane, and the sequence of building blocks in the DNA strand can be ‘read’ as it passes through.
Although attempts have been made to apply the same nanopore technology to proteins, it has not yet been able to carry a lengthy protein through the tiny hole in the same manner that a DNA strand can.
Image Credit: Christoph Burgstedt/Shutterstock.com
Maglia, “It is like cooked spaghetti. These long strands want to be disorganized; they do not want to be pushed through this tiny hole.”
Single-stranded DNA is similar to cooked spaghetti in appearance, but it can be tugged through with an electric field since DNA is electrically charged. Proteins, on the other hand, have a lesser charge and can carry either a positive or negative charge.
“Proteins and DNA are different so the technology needs to be adapted,” Maglia stated.
Going with the Flow
Maglia employed an electric field to draw a solution of electrically charged particles (ions) through a nanopore to transport a protein through it. When this happens, they drag the protein along with them. Making this work was not easy, as Maglia explained.
Maglia further noted, “We didn’t know whether the flow would be strong enough. Furthermore, these ions want to move both ways, but by attaching a lot of charge on the nanopore itself, we were able to make it directional.”
Maglia designed a system with the highest possible flow without the use of proteins. Computer simulations were done in partnership with researchers from the University of Rome Tor Vergata, which demonstrated that the force of this flow on a protein was equivalent to the force of an electric field on DNA.
Maglia then tried it on a challenging protein: one with a lot of negative charges, which causes it to desire to go in the opposite direction of the flow. Even yet, the flow was sufficient to draw the protein through the nanopore.
Maglia added, “Previously, only easy to thread proteins were analyzed. But we gave ourselves one of the most difficult proteins as a test. And it worked! This proves that there is no fundamental limitation to sequencing proteins anymore.”
“With this latest research result, we have the missing piece that we needed to make protein sequencing happen,” Maglia concluded.
Source:
Journal reference:
Sauciuc, A., et al. (2023). Translocation of linearized full-length proteins through an engineered nanopore under opposing electrophoretic force. Nature Biotechnology. doi.org/10.1038/s41587-023-01954-x