Reviewed by Danielle Ellis, B.Sc.Nov 7 2023
Regulatory T cells, or Tregs, represent a specialized class of immune cells dedicated to quelling immune responses and safeguarding the body against self-attack.
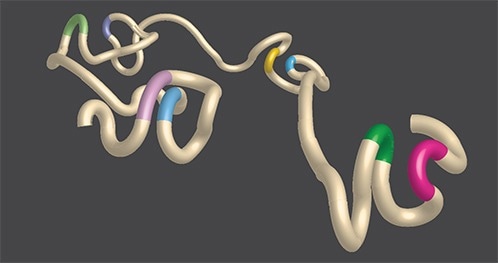 A rendering of chromatin architecture that demonstrates DNA loops. The stretch of DNA (tan) is accented with colored portions of genetic code (green and pink, blue and yellow, light green and purple, and light purple and light blue) that are brought closer together by the formation of loops. Click here for a high-resolution image. Image Credit: Salk Institute.
A rendering of chromatin architecture that demonstrates DNA loops. The stretch of DNA (tan) is accented with colored portions of genetic code (green and pink, blue and yellow, light green and purple, and light purple and light blue) that are brought closer together by the formation of loops. Click here for a high-resolution image. Image Credit: Salk Institute.
Deciphering the mechanisms governing Treg function is pivotal in elucidating strategies for leveraging their potential in combating cancer or averting autoimmune reactions.
The intricate conduct of these cells is intricately tied to the configuration of chromatin—the three-dimensional structure of chromosomes—and the accessibility of genes to proteins, such as Foxp3, a factor that actively fosters the development of regulatory T cells.
In a groundbreaking revelation, Professors Ye Zheng and Jesse Dixon at the Salk Institute have ascertained the indispensable role of Foxp3 in shaping the distinctive chromatin architecture of regulatory T cells. This architectural transformation, in turn, bolsters their ability to execute immune-suppressive functions effectively.

Image Credit: boommaval/Shutterstock.com
The study was published in the journal Nature Communications on November 6th, 2023.
Regulatory T cells are the peacekeepers in our body. Having regulatory T cells telling other cells to calm down is crucial in maintaining a healthy body. Fully understanding the influence of Foxp3 on how these peacekeepers develop teaches us about how our immune system functions—and dysfunctions in disease.”
Ye Zheng, Study Co-Senior Author and Professor, Salk Institute for Biological Studies
Altering a cell's identity, such as transforming an infection-fighting T cell into a regulatory T cell, presents a formidable challenge. The instructions for constructing a cell are encrypted within DNA strands, ensconced within proteins and RNA, and intricately wound into a three-dimensional framework known as chromatin.
Modifications to this three-dimensional architecture wield a profound influence over a cell's identity, as they can either reveal or shield segments of the genetic code responsible for governing the entire cell's behavior.
While the scientific community has long recognized the pivotal role of Foxp3 in regulatory T cell development, its function was traditionally regarded as a binary switch for activating regulatory T cell genes. Yet, Dr. Zheng, a renowned authority on regulatory T cells, believed that this perspective on Foxp3 as a mere genetic on-off switch failed to capture the full scope of its involvement.
Given the complexity and far-reaching impact of chromatin architecture on cellular identity, Dr. Zheng enlisted the expertise of Dr. Dixon, a specialist in chromatin architecture, to investigate the intricate relationship between Foxp3 and regulatory T cells at a higher, structural level.
In their research, the scientists charted the three-dimensional chromatin architecture of regulatory T cells to assess whether Foxp3 induced alterations in this architecture, thereby revealing the genes essential for these cells’ functions.
To discern the distinctive connection between Foxp3 and regulatory T cells, they drew comparisons between the chromatin architecture of regulatory T cells and that of another T cell subtype known as effector T cells. Dr. Zheng explained that effector T cells represent the antithesis of regulatory T cells, as they initiate attacks and instruct other immune cells to engage in combat.
During their examination of the architectural distinctions between regulatory and effector T cells, the researchers observed the existence of numerous exclusive Foxp3 binding regions specific to regulatory T cells. This finding underscored the exceptional and intimate connection between Foxp3 and the immune cells tasked with maintaining peace within the body.
Regulatory and effector T cells follow an almost identical route of differentiation until Foxp3 gets involved. Comparing regulatory and effector T cells gave us a clear picture of Foxp3’s impact on regulatory T cell identity, since Foxp3 is only seen in regulatory T cells.”
Dongsung Lee, Study Co-First Author, Salk Institute for Biological Studies
Furthermore, the researchers discovered distinctive chromatin architecture characteristics known as DNA loops within regulatory T cells.
They observed a physical proximity between genes binding to Foxp3 and genes governing the identity of regulatory T cells. This physical proximity facilitated Foxp3’s capacity to facilitate the expression of genes responsible for shaping cellular identity.
We wanted to see whether Foxp3 was benefiting from DNA loops that the regulatory T cell chromatin structure was already making, or if Foxp3 was in some way creating those characteristic loops. We found that Foxp3 was necessary in creating the loops, and therefore necessary in creating the chromatin architecture unique to regulatory T cells.”
Zhi Liu, Study Co-First Author, Salk Institute for Biological Studies
Foxp3 was found to assume a much more fundamental and expansive role in the development of regulatory T cells than initially anticipated. Previous research had indicated that two Foxp3 proteins interacted in a unique manner to generate these DNA loops.
However, the team’s discoveries indicated that these protein pairs were not indispensable for creating the characteristic loops, raising the possibility that other Foxp3 protein-containing complexes might be involved.
These findings illustrate that Foxp3, beyond its role as a genetic on-off switch, governs substantial genetic structural modifications within regulatory T cells. The presence of Foxp3 directs changes in chromatin architecture, which, in turn, plays a pivotal role in steering the functional success of these immune cells dedicated to maintaining peace within the body.
“Now that we know Foxp3 plays a greater role in regulatory T cell function, we may be able to find ways to turn up and down Foxp3 to regulate immunosuppression,” says Dixon, co-senior author of the study.
Dixon added, “If we turn up Foxp3, we could see more immunosuppression, which could treat autoimmunity. If we turn down Foxp3, we could see less immunosuppression, which could be helpful in fighting cancerous tumors, since normally regulatory T cells infiltrate tumors and suppress the action of other immune cells.”
Further investigation is required to unravel the intricate interactions between Foxp3 and other proteins in the formation of DNA loops within regulatory T cells.
As scientists continue to unveil additional insights into the interplay between Foxp3 and regulatory T cells, they hold the aspiration that Foxp3 may emerge as a prospective target for therapies designed to fine-tune immunosuppression.
Source:
Journal reference:
Liu, Z., et al. (2023) Foxp3 orchestrates reorganization of chromatin architecture to establish regulatory T cell identity. Nature Communications. doi.org/10.1038/s41467-023-42647-y.