A vital set of proteins found in mammals enables cells to operate normally, even in less-than-ideal circumstances.
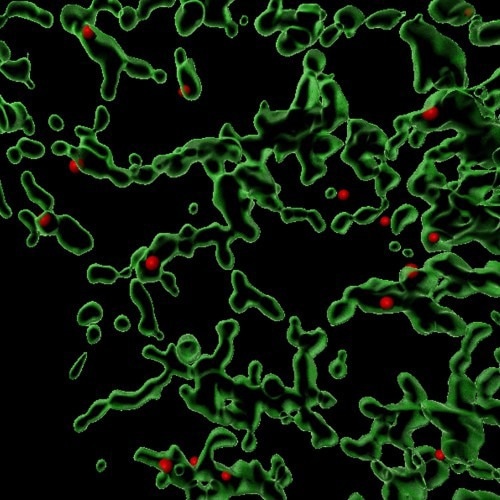 This image is a 3D reconstruction showing sites (in red) where the Coat Protein Complex II (COPII) facilitates the packaging of various proteins within a mammalian cell. The green areas are the endoplasmic reticulum, where protein sorting and trafficking take place. Image Credit: Anjon Audhya
This image is a 3D reconstruction showing sites (in red) where the Coat Protein Complex II (COPII) facilitates the packaging of various proteins within a mammalian cell. The green areas are the endoplasmic reticulum, where protein sorting and trafficking take place. Image Credit: Anjon Audhya
With the aid of cutting-edge genome editing and cell imaging techniques, researchers at the University of Wisconsin–Madison have started to understand how this group of proteins carries out its vital function. Eventually, the discovery may aid in the better understanding and creation of novel medicines for illnesses like diabetes, cancer, and immunological dysfunction.
The research team, under the direction of Anjon Audhya, a Professor at the Department of Biomolecular Chemistry, aimed to get further insight into the operation of the Coat Protein Complex II, or COPII. Roughly one-third of all the proteins that are active in mammalian cells are transported by the highly significant COPII protein group.
Three scientists were given the 2013 Nobel Prize in Physiology or Medicine for their work characterizing the sorting and movement of proteins within cells, and COPII was one of their subjects. Some of those findings are expanded upon in this latest study.
Mammalian cells contain millions of proteins, each with a diverse range of functions. To carry out their complex and precise biological functions, proteins must be transported effectively to their correct locations by cells. Although COPII has been shown to be a crucial component of this process in earlier studies, the precise mechanism by which this collection of proteins packages and moves other proteins throughout cells has not been documented.
To achieve this, Audhya and colleagues employed the CRISPR/Cas9 genome editing technique to modify several proteins involved in regulating the traffic flow within cells, including some that comprise the COPII complex, by adding a tag that could be chemically attached to a vivid fluorescent dye. The scientists could track the proteins' movements within living cells by using the tag.
The researchers achieved a first by tracking how COPII facilitates the delivery of cellular proteins, including ones intended for other locations, to their intended locations using a technique known as lattice light-sheet imaging.
In a recent publication in the journal Nature Communications, the team detailed their progress. Audhya compared it to the postal service. Though they had never followed these employees as they sorted packages via the cell's distribution and delivery mechanisms, researchers understood that COPII performed tasks similar to those of postal workers who pick up and deliver packages.
We can now see that envelope in the mailbox, see how the mail carrier comes to the mailbox to pick up the letter and then drive away,”
Anjon Audhya, Senior Associate Dean, Basic Research, Biotechnology and Graduate Studies, School of Medicine and Public Health, University of Wisconsin–Madison
The researchers found that, under typical circumstances, this delivery procedure takes 45 to 60 seconds on average. But this process slows down considerably when cells receive inadequate nutrition, which can happen occasionally due to certain illnesses and environmental factors, at least until the cells have time to adjust.
Audhya and his associates conducted a number of tests before identifying a single protein, Sec23, that could aid in the restoration of COPII's trafficking pathway following a disruption. The scientists observed a shift in how cells moved proteins when they raised the amount of Sec23 synthesized inside the cells.
Audhya says, “Something we never anticipated when we started this work is that Sec23 seems to be the central player in regulating the function of the COPII complex.”
Determining the trigger that causes Sec23 to enhance COPII function may have consequences for various medical conditions. For example, cancer cells frequently proliferate in nutrient-starved conditions, partly due to increased production of specific growth-promoting proteins. Comprehending the molecular processes behind this characteristic may reveal novel targets for therapeutic interventions.
Beyond that, a clearer picture of how cells correctly assemble and distribute proteins might contribute to the fundamental knowledge of how cells should function and what can go wrong in diseases, including cancer, Type 2 diabetes, immunological disorders, and neurodegenerative problems.
Audhya mentions, “Understanding these fundamental processes and the regulatory systems that exist in cells can ultimately pave the way to developing more rational approaches to disease intervention.”
Source:
Journal reference:
Kasberg, W., et.al., (2023). Nutrient deprivation alters the rate of COPII subunit recruitment at ER subdomains to tune secretory protein transport. Nature Communications. doi.org/10.1038/s41467-023-44002-7.