The central nervous system requires a tightly controlled environment for the neurons in the brain to function properly and be able to process information. The blood-brain barrier, which is maintained by specific brain endothelial cells lining blood vessel inner walls, controls the exchange of chemicals between the neurological and circulatory systems.
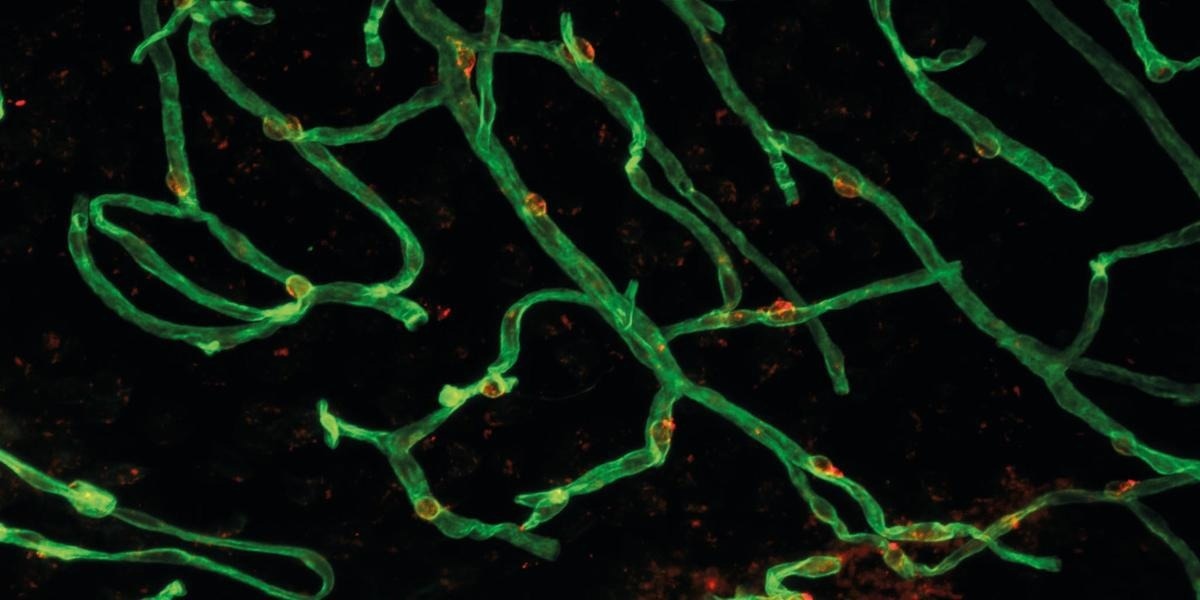 Stained mouse microvessels under the fluorescence microscope (green: vascular endothelium, red: cell nuclei). Image Credit: Dichgans LabImage
Stained mouse microvessels under the fluorescence microscope (green: vascular endothelium, red: cell nuclei). Image Credit: Dichgans LabImage
Previous research has demonstrated that a person's ability to perform a number of functions that rely on these cells, including maintaining the integrity of the blood-brain barrier and controlling the flow of blood to the brain, declines with age. This dysregulation is a crucial contributing factor to medical illnesses like dementia and strokes because it causes a dysfunction of the brain vasculature.
Still, little is known about the molecular alterations underlying this loss of function. Researchers perform molecular profiling studies to examine the many components of brain endothelial cells and compile their results into big databases to further mechanistic understanding.
The transcriptome that is to say, the RNA contained in endothelial cells has since been quite comprehensively mapped, and what has been lacking is corresponding data on the complete set of proteins in the cells, the proteome.”
Martin Dichgans, Professor and Director, Institute for Stroke and Dementia Research, University of Munich Hospital
Dichgans is also a Principal Investigator at the SyNergy Cluster of Excellence.
A recent study, which was published in the journal Nature Aging, seeks to fill this knowledge gap and includes significant contributions from researchers at LMU and SyNergy.
Dysregulated Metabolism
To address age-related changes in protein composition, the research team devised a procedure for enriching brain endothelial cells in mice. These protein dynamics were then connected to biological processes by the scientists using an unsupervised (computer-aided) cluster analysis.
Our results show a dysregulation of key molecules involved in the uptake of substances into cells, in receptor recycling, and in the degradation of molecules within specific cellular compartments called lysosomes.”
Martin Dichgans, Professor and Director, Institute for Stroke and Dementia Research, University of Munich Hospital
A notable alteration was the reduction of proteins related to vesicle-mediated transport. Furthermore, the study shows that apolipoprotein E depletion, a protein involved in lipid metabolism, causes an accelerated endothelial aging signature.
The results complement and expand findings from studies on the RNA sequencing of brain endothelial cells during aging, and our proteomic approach captures processes that are not detected at the RNA level.”
Martin Dichgans, Professor and Director, Institute for Stroke and Dementia Research, University of Munich Hospital
All things considered, the study provides a framework for comprehending significant endothelial signaling pathways during aging and acts as a database for the next investigations into the function of brain endothelium. To facilitate future use, the researchers are providing their data on the age-related protein abundance of the mouse endothelium in a publicly accessible database.
Source:
Journal reference:
Völgyi, T, K., et al. (2024) Proteomics of mouse brain endothelium uncovers dysregulation of vesicular transport pathways during aging. Nature Aging. doi.org/10.1038/s43587-024-00598-z