Comprehending the configuration of proteins is essential for deciphering their roles and creating medications that specifically target them. By understanding protein dynamics and functions, a group of academics at Brown University have devised a method for quickly predicting various protein configurations using machine learning.
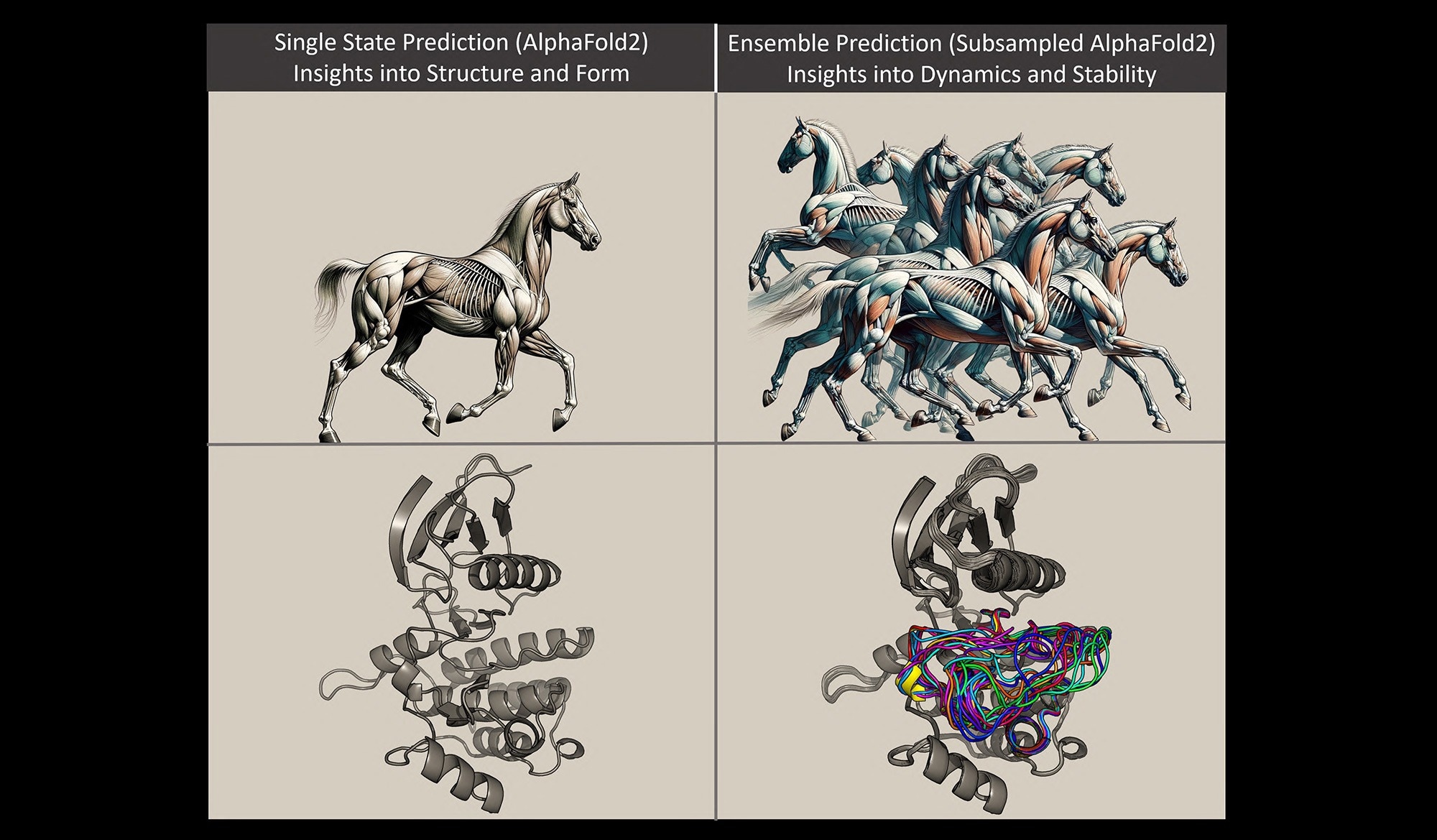 In the same way that multiple snapshots of a galloping horse provide information about how the horse moves, multiple snapshots of a protein changing shape can improve scientific understanding of that protein’s structure and function. Image courtesy of Gabriel Monteiro da Silva.
In the same way that multiple snapshots of a galloping horse provide information about how the horse moves, multiple snapshots of a protein changing shape can improve scientific understanding of that protein’s structure and function. Image courtesy of Gabriel Monteiro da Silva.
The research was published in the journal Nature Communications.
According to scientists, the method is precise, quick, affordable, and has the potential to transform drug development completely by identifying a large number of additional targets for novel therapeutics.
For instance, targeted cancer therapy aims to specifically target proteins that regulate the growth, division, and metastasis of cancer cells. Understanding cell proteins in sufficient detail to identify targets has proven to be a difficulty for structural biologists, according to research author Gabriel Monteiro da Silva, a Brown Ph.D. Candidate in Molecular Biology, Cell Biology, and Biochemistry.
Monteiro da Silva models the dynamics of proteins using computational techniques and searches for ways to enhance existing techniques or develop new techniques that are most effective in certain contexts. He collaborated on this work with Brenda Rubenstein, an Associate Professor of Chemistry and Physics, and other academics at Brown University to test an A.I.-driven computational technique known as AlphaFold2.
Although Monteiro da Silva claimed that AlphaFold2's accuracy had changed the prediction of protein structures, the technique has drawbacks: It only enables scientists to simulate proteins in a static state at a certain moment in time.
During most cellular processes, proteins will change shape dynamically, to match protein targets to drugs to treat cancer and other diseases, we need a more accurate understanding of these physiological changes. We need to go beyond 3D shapes to understand 4D shapes, with the fourth dimension being time. That is what we did with this approach.”
Monteiro da Silva Ph.D., Department of Molecular and Cell Biology and Biochemistry, Brown University
Monteiro da Silva explained protein models with the help of an analogy involving a horse. Protein molecules conform into varied forms because of the bonding arrangements of their constituent atoms, just as the arrangement of the horse's muscles and limbs creates different shapes depending on whether the horse is standing or galloping.
Consider the protein like a horse, suggested Monteiro da Silva. A standing horse model was predicted using earlier techniques. Although it was correct, it provided little information on the behavior or appearance of the horse when it was not standing.
In this work, the scientists were able to control the protein's evolutionary signals by using AlphaFold2 to quickly forecast a variety of protein conformations and the frequency with which those structures will populate.
By using the analogy of a horse, the new technique enables researchers to rapidly forecast many images of a horse galloping. This enables them to see the changes in the horse's muscular structure as it moves and compare those structural variations.
If you understand the multiple snapshots that make up the dynamics of what is going on with the protein, then you can find multiple different ways of targeting the proteins with drugs and treating diseases.”
Brenda Rubenstein, Associate Professor, Department of Chemistry, Brown University
Rubenstein clarified that the team's investigation was centered on a protein for which several medications had been created. However, she claims that for a long time, nobody could figure out why some medications worked and others did not.
It all came down to the fact that these specific proteins have multiple conformations, as well as to understanding how the drugs bind to the different conformations, instead of to the one static structure that these techniques previously predicted; knowing the set of conformations was incredibly important to understanding how these drugs functioned in the body.”
Brenda Rubenstein, Associate Professor, Department of Chemistry, Brown University
Accelerating Discovery Time
The researchers noted that existing computational methods are cost- and time-intensive.
Monteiro da Silva said, “They are expensive in terms of materials, in terms of infrastructure; They take a lot of time, and you cannot do these computations in a high throughput kind of way, I am sure I was one of the top users of GPUs in Brown’s computer cluster. On a larger scale, this is a problem because there is a lot to explore in the protein world: how protein dynamics and structure are involved in poorly understood diseases, in drug resistance, and emerging pathogens.”
The scientists explained how Monteiro da Silva had previously used physics to study protein conformations and dynamics for three years. The innovative A.I.-powered method they used reduced the discovery time to a matter of hours.
Rubenstein said, “So you can imagine what a difference that would make in a person’s life: three years versus three hours, and that is why it was very important that the method we developed should be high-throughput and highly efficient.”
The research team is improving their machine learning methodology to make it more accurate, generalizable, and beneficial for various applications.
Source:
Journal reference:
da Silva, M. G., et al. (2024) High-throughput prediction of protein conformational distributions with subsampled AlphaFold2. Nature Communications. doi.org/10.1038/s41467-024-46715-9