Proteins engage in intricate molecular movements to fulfill their biological roles. The NMDAR protein, crucial for brain function, performs a particularly complex dance. Its precise movements, influenced by binding with glutamate and glycine, are essential for its role as an ion channel.
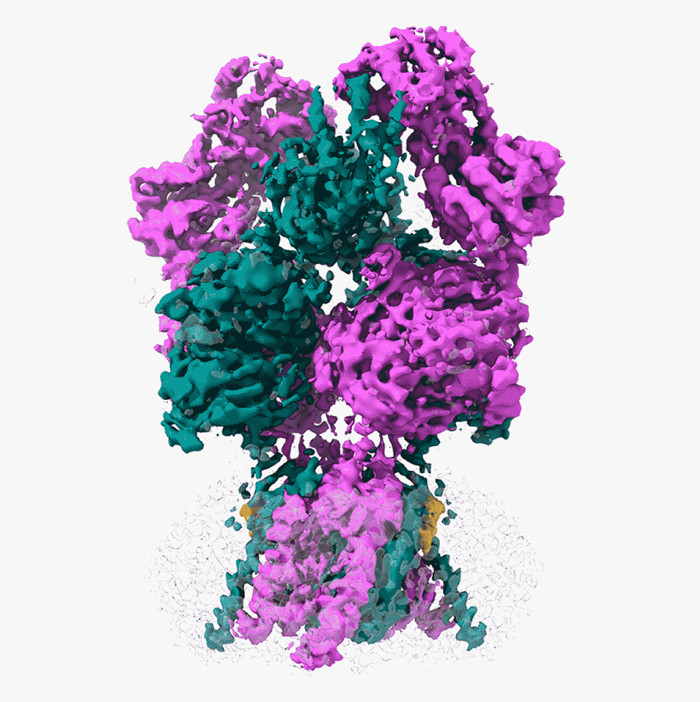 A 3D rendering of the NMDAR protein, GluN1-2B, in its open formation. Image Credit: Cold Spring Harbor Laboratory
A 3D rendering of the NMDAR protein, GluN1-2B, in its open formation. Image Credit: Cold Spring Harbor Laboratory
When the protein completes its “choreography,” it opens, allowing the flow of ions that underlie cognitive processes like memory formation. Disruptions to this delicate dance can lead to neurological disorders.
The issue is that, up until now, scientists have been unable to decipher the final step in NMDAR's routine. Hiro Furukawa, a Professor at Cold Spring Harbor Laboratory, and his colleagues have figured out how NMDAR rotates into an open formation, a crucial dance move. Stated differently, they have mastered the NMDAR "Twist."
Furukawa and his colleagues employed a method known as electron cryo-microscopy (cryo-EM), which freezes and visualizes proteins in action, to record this crucial step. Initially, the group needed to figure out how to image GluN1-2B, a kind of NMDAR, while it was in its open position. Furukawa thus teamed up with Dennis Liotta and Stephen Traynelis, two Emory University professors. They found a molecule together that prefers NMDAR in an open position.
It’s not the most stable conformation. There are many pieces dancing independently in NMDAR. They have to coordinate with each other. Everything has to go perfectly to open the ion channel. We need a precise amount of electrical signals at the right time for proper behaviors and cognitions.”
Hiro Furukawa, Professor, Cold Spring Harbor Laboratory
Researchers can observe the exact motion of the NMDAR's atoms during its "Twist," thanks to the cryo-EM pictures. This could eventually result in medication combinations that can retrain NMDARs who have fallen behind. Improved medications that target NMDARs may find use in treating neurological conditions like depression and Alzheimer's disease.
Compounds bind to pockets within proteins and are imperfect, initially. This will allow us and chemists to find a way to fill those pockets more perfectly. That would improve the potency of the drug. Also, the shape of the pocket is unique. But there could be something similarly shaped in other proteins. That would cause side effects. So, specificity is key.”
Hiro Furukawa, Professor, Cold Spring Harbor Laboratory
The brain contains a wide variety of NMDARs. Furukawa's lab has released the first image of the GluN1-3A NMDAR in another recent study. Its dancing moves are surprisingly different. This procedure produces unusual electrical signal patterns.
Source:
Journal references:
Furukawa, H., et al. (2024) Molecular mechanism of ligand gating and opening of NMDA receptor. Nature. doi.org/10.1038/s41586-024-07742-0.
Michalski, K., & Furukawa, H. (2024) Structure and function of GluN1-3A NMDA receptor excitatory glycine receptor channel. Science Advances. doi.org/10.1126/sciadv.adl5952.