A preclinical investigation conducted by Weill Cornell Medicine researchers has shown an unexpectedly important role for an enzyme called PGK1 in the synthesis of chemical energy in brain cells. Increasing brain activity may assist the brain to fend off energy deficiencies that might cause Parkinson's disease, according to the researchers.
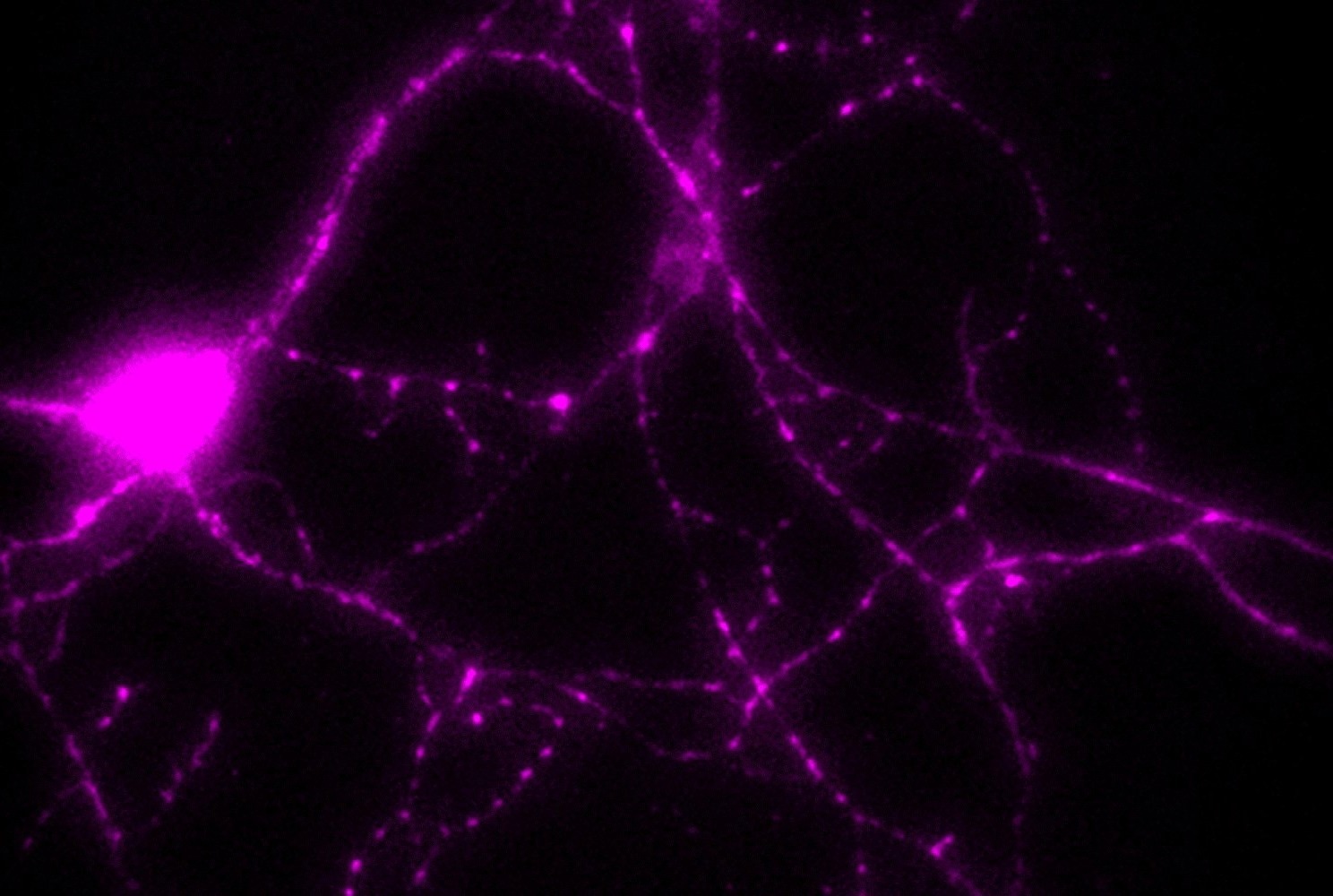 The glycolytic enzyme PGK1 is distributed throughout neurons. This image of an isolated hippocampal neuron from a rat shows that PGK1 is found at nerve terminals (small varicosities) where it helps power synapses. Image Credit: Ryan lab
The glycolytic enzyme PGK1 is distributed throughout neurons. This image of an isolated hippocampal neuron from a rat shows that PGK1 is found at nerve terminals (small varicosities) where it helps power synapses. Image Credit: Ryan lab
The study, which was published in the journal Science Advances, provided proof that PGK1 is a “rate-limiting” enzyme in the axons output-signaling branches of the dopamine neurons damaged by Parkinson's disease when it comes to the creation of energy.
Accordingly, a small increase in PGK1 activity can have a significant impact on replenishing the neuronal energy supply under low-fuel situations. The researchers also demonstrated that this could stop the axon malfunction and degeneration typically observed in an animal model of Parkinson’s disease.
Our findings show that PGK1 can really make a big difference in Parkinson’s disease, in ways we did not anticipate. I am very optimistic that this line of research has the potential to generate new Parkinson’s treatments.”
Dr. Timothy Ryan, Study Senior Author and Tri-Institutional Professor, Biochemistry, Weill Cornell Medicine
Dr. Alexandros Kokotos was the study's primary author and Postdoctoral Researcher at the Ryan Laboratory.
About one million Americans suffer from Parkinson's disease, which is the second most common neurological illness after Alzheimer's. Key populations of dopamine-producing neurons are affected by the disease, which eventually results in their death by first weakening their synapses or connections to other neurons.
Movement impairments, sleep issues, and, ultimately, dementia are the disease's indications and symptoms. Present therapies deal with symptoms but do not reverse the progression of the illness.
Numerous studies over the years have shown that Parkinson's disease, which affects neurons with extremely high energy requirements, may be caused by a breakdown in the neuronal energy supply. Nevertheless, scientists have not yet discovered a viable energy-related target for medical intervention.
Recent research has brought renewed attention to PGK1 by demonstrating that terazosin, an FDA-approved medication used to treat prostate enlargement, also increases PGK1's activity in producing energy and improves several animal models of Parkinson's disease.
However, terazosin's modest ability to increase PGK1 activity in these experiments raises questions about how it works. In a retrospective investigation of human subjects, terazosin was found to dramatically lower the incidence of Parkinson's disease, providing more evidence for the drug's potential involvement in enhancing brain protection.
Pharma companies have been skeptical that this weak enhancement of PGK1 can explain these benefits in Parkinson’s models.”
Dr. Timothy Ryan, Study Senior Author and Tri-Institutional Professor, Biochemistry, Weill Cornell Medicine
Sensitive tests used in Dr. Ryan's group's new work helped resolve this matter by clarifying PGK1's function as an energy producer in neurons.
The researchers demonstrated the significance of this function, finding that when glucose levels are low, a substance that PGK1 aids in converting to fundamental chemical energy units even a slight increase in PGK1 activity, such as that which terazosin offers, is sufficient to maintain axon function. Low-glucose conditions brought on by known gene alterations linked to Parkinson's disease were included in the tests.
The group also discovered something unexpected about a protein known as DJ-1, whose deficiency due to mutation is another recognized hereditary etiology of Parkinson's disease. DJ-1 is a “chaperone” that is supposed to shield neurons by avoiding the accumulation of dangerous proteins.
Nonetheless, the group discovered that DJ-1 functions as PGK1's close associate and unanticipated energy supplier; in fact, DJ-1 is essential to the advantages of PGK1 enhancement.
According to Dr Ryan, the findings support the idea that Parkinson's disease is generally caused by an energy supply deficit in the most vulnerable dopamine neurons, which is brought on by ageing, genetics, and environmental factors. This deficit may be reversed, and the disease process may be blocked by modestly increasing the activity of PGK1, one enzyme.
Now I can say I’m confident that this enzyme is what should be targeted. Given the positive impact of terazosin in protecting against Parkinson’s in humans and the fact that this drug was never optimized for PGK1 enhancement, it is exciting to consider the possible clinical impact of new drugs that, compared with terazosin, can enhance PGK1 activity more potently and selectively.”
Dr. Timothy Ryan, Study Senior Author and Tri-Institutional Professor, Biochemistry, Weill Cornell Medicine
Source:
Journal reference:
Kokotos, A., et al. (2024) Phosphoglycerate kinase is a central leverage point in Parkinson’s disease–driven neuronal metabolic deficits. Science Advances. doi.org/10.1126/sciadv.adn6016.