A group led by Dr. Elena Cano at the Integrative Vascular Biology Lab of Professor Holger Gerhardt at the Max Delbrück Center in Berlin has discovered how new arteries develop in the heart.
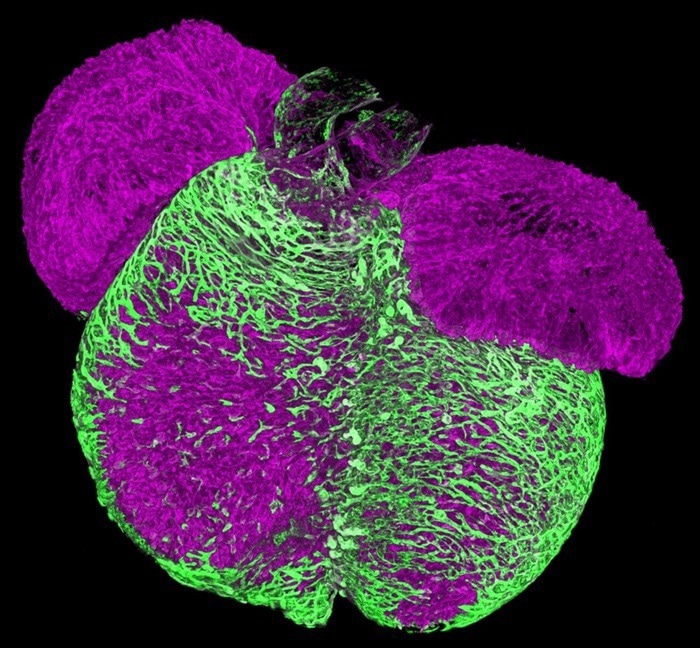 A 3D image of an embryonic mouse heart. Endothelial cells vascularize the heart muscle walls (shown in green). This process establishes the coronary vasculature that nourishes the heart muscle. Image Credit: Max Delbrück Center
A 3D image of an embryonic mouse heart. Endothelial cells vascularize the heart muscle walls (shown in green). This process establishes the coronary vasculature that nourishes the heart muscle. Image Credit: Max Delbrück Center
The research, which was published in “Circulation Research,” closes a significant knowledge gap about the pathophysiology of coronary arteries and may enhance the efficacy of therapies intended to repair myocardial damage resulting from heart attacks and strokes, which are the world's greatest causes of mortality and disability. DZHK provided funding for the project.
Heart muscle tissue may perish as a result of a heart attack or stroke. The heart's blood flow is subsequently reduced, resulting in irreversible impairment. While some tissue can regenerate its blood supply on its own by forming new blood vessels, this process is frequently insufficient to restore blood supply fully. Current treatments concentrate on symptom management and slowing heart disease.
Researchers have tried many different techniques to encourage the growth of new blood arteries in injured heart tissue. Cano claims that they have not succeeded in creating a mature, stable vascular network that enhances cardiac function. One of the main disadvantages is the lack of greater knowledge of the complex cellular, chemical, and structural cues that vascular cells utilize to construct a hierarchical blood artery network.
Applying Single-Cell Sequencing to the Problem
Cano found a certain kind of pre-arterial cell in her samples while researching the vascularization of tissues, and this cell type seemed to be crucial for the development of new arteries. Previous studies have already reported on these pre-arterial cells. But Cano wanted to use modern technologies to analyze them.
The researchers demonstrated that these cardiac pre-arterial cells originate from “tip cells,” which are specialized cells that sense environmental cues to drive growing vessels into specific directions. They did this by analyzing the transcriptome of cardiac cells taken from mice at different stages of development using single-cell sequencing.
Additionally, the group uses 3D spatiotemporal mapping to validate their findings. Furthermore, contrary to what is currently believed about how arteries form, they demonstrated that these pre-arterial cells were already “marked” to develop into arterial cells.
It had been considered that new arteries form their distinctive properties, such as length and diameter, based entirely on mechanical cues, such as the velocity of the fluid passing through them.
This study shows that pre-arterial cells already have characteristics of arteries before any fluid flows through them.”
Dr. Elena Cano, Max Delbrück Center
Additionally, the researchers reexamined previously published single-cell data from human embryonic heart tissue that had been collected by UK researchers, comparing it to their data from heart attack-damaged mouse heart tissue. They discovered that the mechanism by which new arteries formed in the human embryonic tissue was the same as that which occurred in mice after heart attack damage.
We show that not only is this mechanism conserved between mice and humans during development, but it also persists throughout life and becomes activated after a heart attack.”
Dr. Elena Cano, Max Delbrück Center
Implications for Treatment
Cano argues that by comprehending how coronary arteries naturally build and repair, therapies that activate these regenerative pathways may be developed, potentially correcting heart muscle injury.
Now we know that it is not only flow that signals vascular endothelial cells to become arteries, but that tip cells transition into pre-arterial cells to eventually become arteries, Surprisingly, we find that not all tip cells have the capacity to form arteries, raising the prospect of selectively increasing this pool of cells for therapeutic purposes.”
Holger Gerhardt, Professor, Max Delbrück Center
Cano added, “This is a step forward, It is a new mechanism that we can potentially tweak during regeneration to see whether we can form new arteries for optimal restoration of blood supply.”
Source:
Journal reference:
Cano, E., et al. (2024) Intramyocardial Sprouting Tip Cells Specify Coronary Arterialization. Circulation Research. doi.org/10.1161/circresaha.124.324868.