Researchers at the Institute for Integrated Cell-Material Sciences (WPI-iCeMS) at Kyoto University have discovered new information regarding how cells control the distribution of lipids in their cell membrane. These lipids, referred to as phospholipids, are layered in a bilayer of membranes that control specific molecules' admission and departure to preserve a steady internal environment.
 In normal cells (left), calcium activates the Tmem63b/Slc19a2 complex, leading to controlled phospholipid scrambling, along with Kcnn4 activation. In Tmem63b mutant (derived from human patients) cells (right), continuous phospholipid scrambling occurs without calcium stimulation. Image Credit: H Niu, M Maruoka, Y Noguchi, H Kosako, and J Suzuki
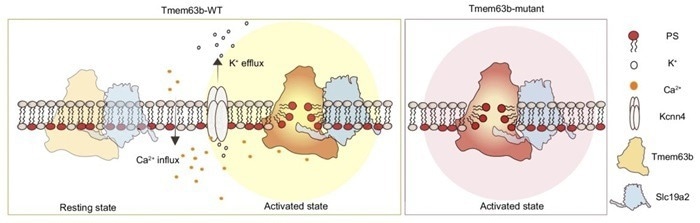
In normal cells (left), calcium activates the Tmem63b/Slc19a2 complex, leading to controlled phospholipid scrambling, along with Kcnn4 activation. In Tmem63b mutant (derived from human patients) cells (right), continuous phospholipid scrambling occurs without calcium stimulation. Image Credit: H Niu, M Maruoka, Y Noguchi, H Kosako, and J Suzuki
The distribution of phospholipids in cell membranes is typically non-uniform, with certain types remaining on the inside and others on the outside. Cells must, however, swiftly alter this distribution in response to internal or external cues.
Certain phospholipids may become visible to the exterior of the cell due to phospholipid scrambling, the movement of phospholipids from one side of the membrane to the other. Numerous processes, such as blood coagulation and the elimination of undesirable cells, depend on this exposure.
Current research, reported in the journal Nature Communications, has found protein complexes crucial to this process.
We discovered that when calcium is incorporated into cells, a specific protein complex, including the ion channel Tmem63b and the vitamin B1 transporter Slc19a2, triggers phospholipid scrambling.”
Jun Suzuki, Professor and Study Lead, Kyoto University
When calcium enters the cell, it acts as a signal that can trigger a number of different biological functions, including phospholipid scrambling and ion channel gating.
When Tmem63b was deleted, the cells lost calcium-induced phospholipid scrambling activity. Conversely, certain genetic mutations in the Tmem63b gene linked to diseases like epilepsy and anemia lead to continuous activation of phospholipid scrambling, even without calcium stimulation.”
Han Niu, Study First Author, Kyoto University
The researchers also discovered that this mechanism is influenced by Kcnn4, a potassium channel that is triggered by calcium. Phospholipid scrambling was decreased when Kcnn4 or Slc19a2 were absent. This demonstrates how Kcnn4, Slc19a2, and Tmem63b cooperate to control phospholipid scrambling.
Other proteins implicated in phospholipid scrambling were found in earlier research by Suzuki and colleagues, but these findings were insufficient to account for all occurrences. The new finding demonstrates that, in contrast to the other proteins, which function as pairs consisting of two copies of the same protein, Tmem63b and Slc19a2 function as a pair bonded together to initiate this process.
The researchers also discovered that variations in the plasma membrane tension within the cell may aid in the activation of the Tmem63b/Slc19a2 complex. A cell may shrink as a result of calcium entering the cell and potassium ions leaving by Kcnn4.
The cell membrane tension may alter as a result of this shrinkage, which will make it easier for Tmem63b to activate when intracellular calcium levels rise. This activation mechanism may account for the phospholipid scrambling that red blood cells and neurons use to respond to changes in their environment.
The researchers anticipate that new treatments for conditions like epilepsy and anemia, which are caused by disruptions in phospholipid scrambling, will result from their discovery.
Source:
Journal reference:
Niu, H., et al. (2024) Phospholipid scrambling induced by an ion channel/metabolite transporter complex. Nature Communications. doi.org/10.1038/s41467-024-51939-w.