In a recent discovery, a study team from Kyushu University in Japan has shed light on how the systems defend against illness and injury by identifying a calcium-based mechanism that is essential to the removal of dead cells.
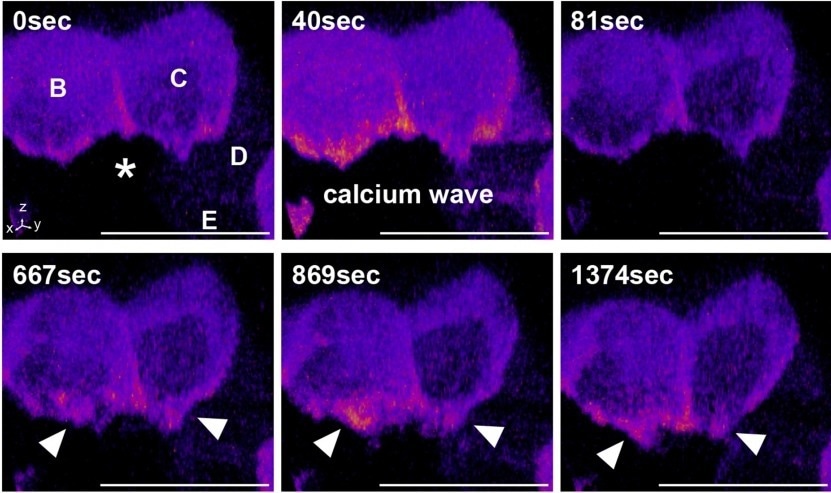 In this image, the cell marked with an asterisk was damaged using a focused laser, triggering apoptosis. After an initial increase in general calcium levels around the dying cell (calcium wave), a sustained increase in calcium levels was observed at the interface between the apoptotic cell and the surrounding cells (marked B and D). This new mechanism is essential for expelling the apoptotic cell and preserving the integrity of the epithelium. Image Credit: Kyushu University
In this image, the cell marked with an asterisk was damaged using a focused laser, triggering apoptosis. After an initial increase in general calcium levels around the dying cell (calcium wave), a sustained increase in calcium levels was observed at the interface between the apoptotic cell and the surrounding cells (marked B and D). This new mechanism is essential for expelling the apoptotic cell and preserving the integrity of the epithelium. Image Credit: Kyushu University
The team used genetically engineered epithelial tissue cultures, molecular markers, and advanced imaging techniques to reveal how calcium ion levels are critical for the effective removal of dying or apoptotic cells from epithelial tissues (cells lining the body surface). Their study was published in the journal Current Biology.
Sheets of epithelial cells cover the surfaces of human bodies, including the skin and internal organs, and serve as essential barriers. Neighboring cells rapidly unite to push these injured and dying (apoptotic) cells out of the way and close any openings that would allow foreign chemicals to enter and possibly cause infections or inflammation.
Even though this intricate process is necessary to preserve a healthy epithelial barrier, its precise underlying mechanism has not yet been fully understood.
In addition to partners from the University of Tokyo and the Health Sciences University of Hokkaido in Japan, Professor Junichi Ikenouchi of Kyushu University coordinated the study, which included contributions from Dr. Kenji Matsuzawa and lead author Mr. Yuma Cho.
First, the group utilized a concentrated laser to cause individual epithelial cells to undergo apoptosis. They then watched how the surrounding cells responded. After that, they altered the surrounding cells to express specific calcium ion sensors known as GCaMP6, which allowed them to see calcium variations in real-time.
Interestingly, they discovered that the apoptotic cell's neighbors had a sizable jump in calcium levels, especially close to the membrane areas where they interfaced with the dying cell. This fascinating phenomenon was given the term “calcium response in effectors of apical extrusion (CaRE)” by the researchers.
To further understand this recently identified process, the researchers next looked into the function of IP3 receptors, which are internal proteins that aid in the regulation of calcium ion levels.
They discovered that apoptotic cells could not be expelled at all when IP3 receptor function was inhibited or the genes linked to these receptors were eliminated. Advanced electron microscopy was used for additional study, which showed that CaRE is primarily mediated by a certain group of IP3 receptors, especially those that are close to desmosomes.
Desmosomes are essential for preserving the stability and shape of the tissues in the human body because they guarantee that adjacent cells stick closely to one another. The scientists discovered that to cause the contraction of the actomyosin complex, a set of proteins that aids in cell shape and movement and aids in the evacuation of apoptotic cells, IP3 receptors close to desmosomes must be activated.
Our study sheds light on a newfound role of IP3 receptors in desmosomes, the latter of which was previously thought to be involved only in mechanical connections between epithelial cells.”
Junichi Ikenouchi, Professor, Kyushu University
The team points out that because this work was done on cultured cells, more research on the CaRE mechanism is required to find out if it functions in live things as well, if it differs in different organ tissues, and if other components are involved.
This study enhances the understanding of how the body sustains a healthy epithelium—an essential function that is often taken for granted.
Our findings provide valuable insights into understanding diseases caused by epithelial barrier disruption, such as atopic dermatitis and inflammatory bowel disease, and may contribute to the development of new preventive measures and treatments for chronic inflammation.”
Junichi Ikenouchi, Professor, Kyushu University
Source:
Journal reference:
Cho, Y., et al. (2024) A sustained calcium response mediated by IP3 receptor anchoring to the desmosome is essential for apoptotic cell elimination. Current Biology. doi.org/10.1016/j.cub.2024.08.057.