Ben Orlando's research talk at MSU's Department of Biochemistry and Molecular Biology in 2019 laid the groundwork for a team effort that had been developing for decades.
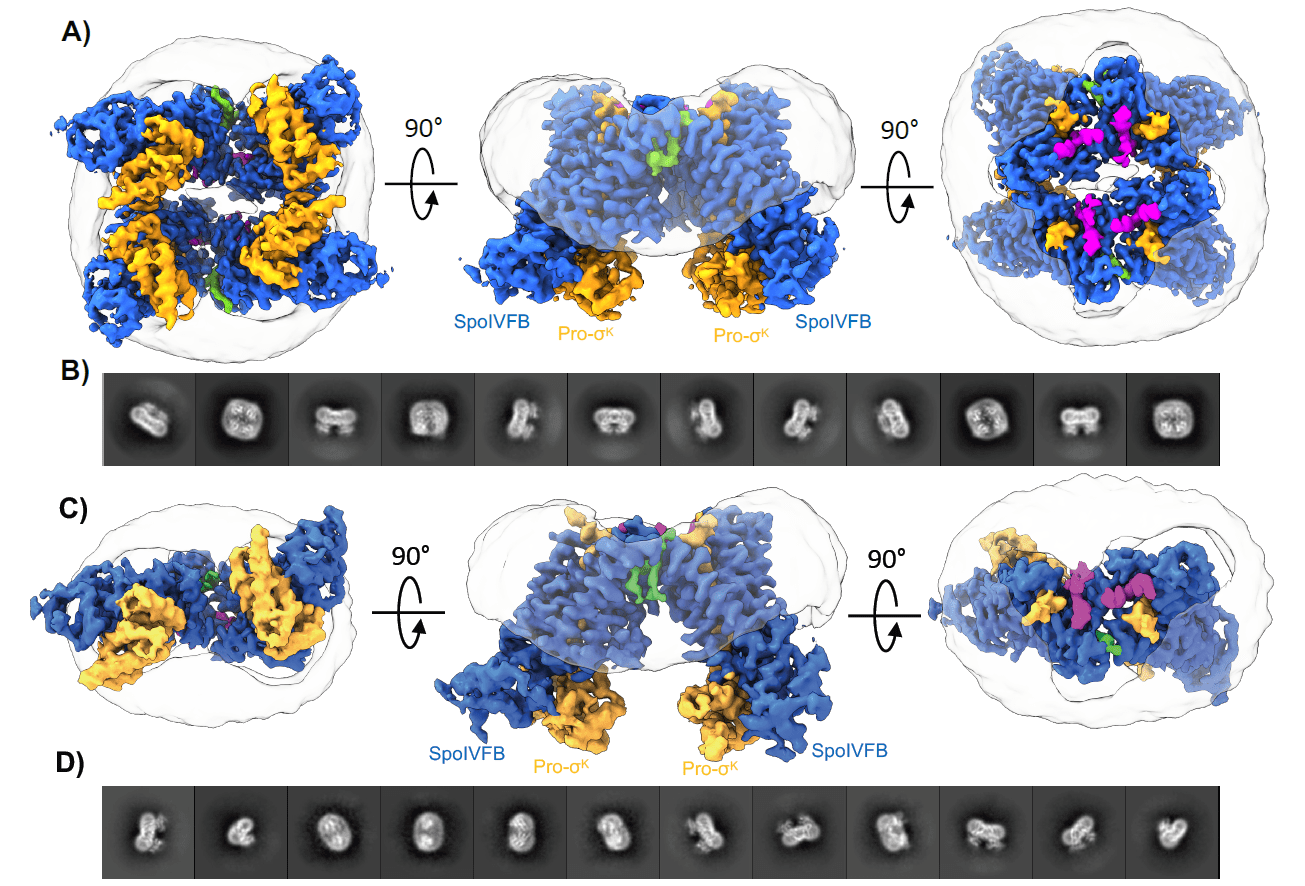 Figure showing the 3D structure of SpoIVFB alongside black and white 2D cryo-EM images. By taking thousands or sometimes millions of images of a sample at different angles, researchers are able to create a highly detailed 3D map. Image Credit: Michigan State University College of Natural Science.
Figure showing the 3D structure of SpoIVFB alongside black and white 2D cryo-EM images. By taking thousands or sometimes millions of images of a sample at different angles, researchers are able to create a highly detailed 3D map. Image Credit: Michigan State University College of Natural Science.
Kroos, who specializes in the genetic and molecular mechanics of bacteria, has long collaborated with colleagues from Sparta to unravel the enigmatic structure of a protein called SpoIVFB.
A member of the intramembrane protease family of specialized enzymes, SpoIVFB is present in the model bacterium Bacillus subtilis, or B. subtilis. In all kingdoms of life, these enzymes aid in the regulation of essential cellular processes.
Notably, SpoIVFB is essential for sporulation, which enables bacteria to endure extreme environments, including heat, radiation, and even space.
Kroos was intrigued to hear about Orlando's proficiency in the cutting-edge science of cryo-electron microscopy, or cryo-EM, as efforts to capture the structure of SpoIVFB have encountered particular difficulties over the years.
“I think Ben and I were on the same wavelength,” Kroos said, who, when Orlando joined the Department of Biochemistry and Molecular Biology, or BMB, in the summer of 2020, contacted him about working together to solve the structure puzzle of SpoIVFB.
“Ben was very optimistic we could make progress on this thing.”
Now, the Orlando and Kroos research groups present the first high-resolution empirically determined structures of SpoIVFB in a study published in the journal Nature Communications.
The scientists discovered that SpoIVFB was attached to its substrate. Substrates are specific molecules that enzymes interact with to produce useful biochemical products, much like a key to a lock.
This study's understanding of a cellular control system present in creatures ranging from bacteria to humans has intriguing ramifications for microbiology, structural biology, enzymology, and human disease.
The discovery also demonstrates how MSU's $15 million upgrade of its cutting-edge cryo-EM equipment is enabling Spartan researchers to push the limits of experimental viability.
Over the last several years this technology has been transformative in membrane protein structural biology.”
Ben Orlando, Department of Biochemistry and Molecular Biology, Michigan State University
MSU hired him as part of its Global Impact Initiative, a campus-wide project aimed at addressing major issues in the fields of education, health, energy, and the environment.
“Cryo-EM allows us to peer into a world that we simply cannot see through any other lens.”
The Last Piece of a Biological Puzzle
Thanks to the researchers' ability to capture the structure of SpoIVFB interacting with its substrate, biochemists now have a better understanding of a crucial biological function.
Through a process called proteolysis, which needs water to break the peptide bonds between amino acids, proteases usually aid in the breakdown or “cleavage” of proteins. This is no easy task, though, for a protease like SpoIVFB that is found in the membrane of a cell.
Going back to the discovery of intramembrane proteases what some call ‘scissors in the membrane’ there was no concept this could happen. The membrane’s lipid bilayer is hydrophobic, and all proteases need water to break peptide bonds. So, the big question became: how does water get in there?”
Emeritus Lee Kroos, Professor, Michigan State University
Using molecular dynamics and cryo-EM imaging, Kroos and Orlando verified the process by which SpoIVFB primes its substrate for cleavage and the path that water may follow to get there.
Orlando and Kroos's cryo-EM structures show that SpoIVFB interacts with the substrate via a process called beta-sheet augmentation. This discovery was the last piece of a huge biological puzzle. All kingdoms of life, from the most basic bacteria to humans, have four kinds of intramembrane proteases. It was known that three of these used beta-sheet augmentation to interact with their substrates.
The MSU team has finally validated the fourth with their most recent discovery.
“These structures link the beta-sheet augmentation mechanism to all four classes of intramembrane proteases, operating as a common mechanism across the kingdoms of life,” Orlando said, who in 2023 was invested a James K. Billman Jr., M.D. Endowed Professor.
Both human health and structural biology benefit greatly from these discoveries.
Cancer, metabolic problems, and neurological diseases have all been related to intramembrane proteases' inability to control cellular processes.
There are many indications that these intramembrane proteases also influence the virulence of important pathogenic bacteria, If we know their structure precisely, we could design antimicrobials for important diseases.”
Emeritus Lee Kroos, Professor, Michigan State University
Kroos, who was recently observed enjoying his retirement fly-fishing on the Pere Marquette River, had an amazing 36-year career at BMB and the Department of Microbiology, Genetics, and Immunology. The team's discoveries are also a suitable capstone to that career.
Kroos said, “Over the past few years, collaborating with Ben has been awesome. We have had wonderful, in-depth conversations on this work it is been a real high note.”
From Atoms to Organisms
Kroos and his fellow BMB scientists first used X-ray crystallography in their years-long effort to characterize SpoIVFB. Here, a crystallized sample is exposed to X-rays, which produce a diffraction pattern that eventually reveals the t3D molecular structure.
However, two recurring challenges were the number of samples needed and the quality of SpoIVFB's crystals.
“SpoIVFB was very resistant to our crystallization efforts, and it was difficult to make significant quantities that were active and could be used,” Kroos explained.
Orlando added, “Coaxing proteins to coalesce into crystals is challenging enough. But when you start talking about membrane proteins like SpoIVFB, you have got even more complexity.”
The Kroos Group, working with BMB colleagues Michael Garavito, Jian Hu, and Michael Feig, continued to make important discoveries during their crystallization attempts, which eventually led to the solution of SpoIVFB's structure.
Through their most recent collaboration, Orlando and Kroos were able to visualize SpoIVFB like never before by avoiding some of the largest bottlenecks in X-ray crystallography. Thanks to cryo-EM, they were able to visualize SpoIVFB like never before. In single-particle electron microscopy, individual protein molecules are imaged in two dimensions using an electron beam. Subsequently, a 3D map of the target protein is created using these 2D pictures.
These same electrons can harm or destroy biological samples, even though they provide atomic-scale resolution photographs of many materials. This obstacle paved the way for the cryogenic uses of cryo-EM, a breakthrough acknowledged with the 2017 Nobel Prize in Chemistry.
Cryo-EM samples are prepared in a thin layer of aqueous buffer and quickly submerged in liquid ethane to create a “glass-like” ice, which prevents biological damage. At the time of freezing, individual particles are locked into different orientations rather than having to be assembled into a crystal lattice for investigation.
Researchers can produce a very thorough 3D map of the sample they are imaging by capturing hundreds or even millions of pictures of it from various perspectives.
“This technology helped get us over that mountain, and as it improves, we will peer deeper and deeper into new areas of biology,” Orlando said,
Orlando is also interested in the implications of MSU's cryo-EM facility expansion for researchers in a variety of fields, including materials science, microbiology, and biochemistry.
“I think the real power of a place like this comes from the people across our campus who can utilize the exact same technology and its breadth of scale. It is the only methodology that I am aware of where we can view it all, from atoms to organisms,” Orlando added.
Source:
Journal reference:
Orlando, M. A., et al. (2024) Substrate engagement by the intramembrane metalloprotease SpoIVFB. Nature Communications. doi.org/10.1038/s41467-024-52634-6.