Reviewed by Danielle Ellis, B.Sc.Nov 29 2024
An important mechanism in fat cells (adipocytes) that allows them to safely enlarge to store energy has been discovered by a team at the Centro Nacional de Investigaciones Cardiovasculares (CNIC), led by Professor Miguel Ángel del Pozo Barriuso, who leads the CNIC's Mechanoadaptation and Caveolae Biology group.
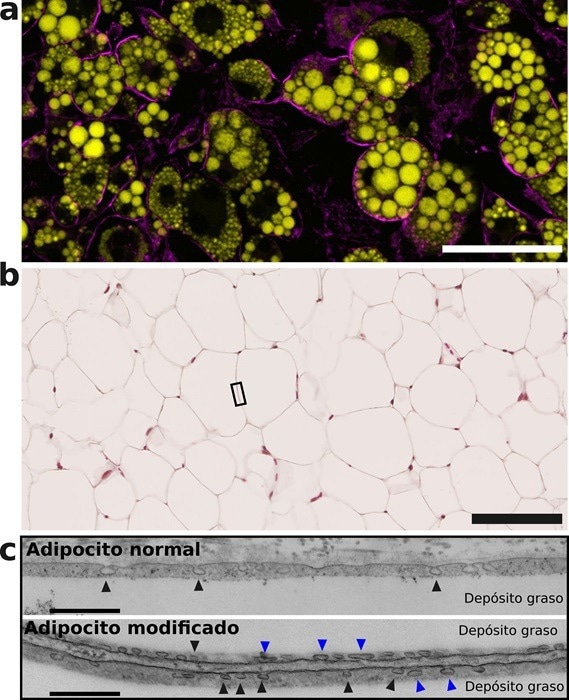 Adipocytes differentiated in vitro, containing fat deposits (yellow) and the Cav-1 protein in the cell membrane (magenta). Scale bar, 100 microns. b, Mouse adipose tissue. Scale bar, 100 microns. c, Electron microscopy images showing regions of adipocyte membrane similar to that indicated by the boxed area in b. Caveolae are visible as membrane invaginations (arrowheads). The genetically modified adipocyte expressing non-phosphorylatable Cav-1 (bottom panel) accumulates caveolae because they cannot flatten in response to the accumulation of fat (blue arrowheads). Scale bar, 500 nm. Image Credit: CNIC
Adipocytes differentiated in vitro, containing fat deposits (yellow) and the Cav-1 protein in the cell membrane (magenta). Scale bar, 100 microns. b, Mouse adipose tissue. Scale bar, 100 microns. c, Electron microscopy images showing regions of adipocyte membrane similar to that indicated by the boxed area in b. Caveolae are visible as membrane invaginations (arrowheads). The genetically modified adipocyte expressing non-phosphorylatable Cav-1 (bottom panel) accumulates caveolae because they cannot flatten in response to the accumulation of fat (blue arrowheads). Scale bar, 500 nm. Image Credit: CNIC
This procedure avoids tissue damage and shields the body from the harmful effects of lipids (fat molecules) building up in the wrong areas. The findings, published in the journal Nature Communications, represent a significant breakthrough in the knowledge of metabolic disorders.
Furthermore, this finding paves the way for the creation of novel treatment approaches to address the serious metabolic and cardiovascular consequences of disorders, including metabolic syndrome, overweight, obesity, and lipodystrophy, which are linked to persistent energy excess.
Adipose tissue is a major factor in determining metabolic health in contemporary civilizations, which are marked by sedentary lifestyles and high-calorie meals. To prevent excess lipids from building up in organs like the liver or in the blood vessel wall (particularly in the heart and brain), where they could cause irreversible harm, adipocytes can grow to store energy in the form of fat.
However, there are risks associated with this process. Overloading adipocytes with fat can cause them to burst, releasing their toxic contents and causing inflammation and changes in metabolism.
The CNIC study looked at how adipocytes adjust to the mechanical stress of expanding to make room for extra fat.
Caveolae are tiny invaginations in the cell membrane that serve as sensors, and the scientists examined their function.
When an adipocyte accumulates fat and its surface is under increased tensile stress, the caveolae flatten, releasing a ‘reservoir’ of the membrane that allows the cell to enlarge without breaking apart. Conversely, when fat reserves diminish, these structures regroup to reduce the excess membrane and restore cellular stability.”
Dr. María Aboy Pardal, Study First Author, Centro Nacional de Investigaciones Cardiovasculares
Caveolae: Not Just a Structural Support
In addition to providing adipocytes with physical protection, caveolae are crucial for regulating cell metabolism.
Miguel Ángel del Pozo Barriuso explained that during adipocyte expansion, “molecular components of these membrane structures travel to other cell compartments, conveying signals that adjust metabolic activity to match the level of energy reserves. This capacity for internal communication makes caveolae key elements for efficient caveolar function.”
Adipocytes become less effective at storing energy, more stiff, and prone to rupture when these structures are missing or broken.
Is an inflammatory reaction that compromises the body’s metabolic health. This phenomenon is linked to conditions such as lipodystrophy, in which the body cannot store fat, leading to severe metabolic and cardiovascular alterations.”
Aboy Pardal, Centro Nacional de Investigaciones Cardiovasculares
According to the CNIC study, the caveolae protein caveolin-1 (Cav-1) plays a crucial role. For caveolae to properly flatten in response to variations in the mechanical tension in the cell membrane, Cav-1 must undergo phosphorylation, which is the chemical addition of a phosphoryl group to a particular amino acid.
The researchers created a transgenic mouse for the study that expresses a genetically modified form of Cav-1 that is incapable of being phosphorylated. This significantly reduces adipocytes' ability to store energy and preserve cellular integrity by preventing them from properly expanding in response to the mechanical tension created by the buildup of lipids.
Lipodystrophy and its dire repercussions are the final result of this straightforward system failing.
These results give us a better understanding of how adipose tissue responds to the mechanical forces associated with energetic excess. In the context of obesity and metabolic syndrome, this protective mechanism is essential for minimizing organismal damage.”
Miguel Ángel del Pozo Barriuso, Professor, Centro Nacional de Investigaciones Cardiovasculares
Source:
Journal reference:
Aboy-Pardal, M. C. M, et al. (2024) Plasma membrane remodeling determines adipocyte expansion and mechanical adaptability. Nature Communications. doi.org/10.1038/s41467-024-54224-y.