Scientists are shining a laser on the energy centers of cancer cells in an attempt to damage these sources of power and cause widespread cancer cell death.
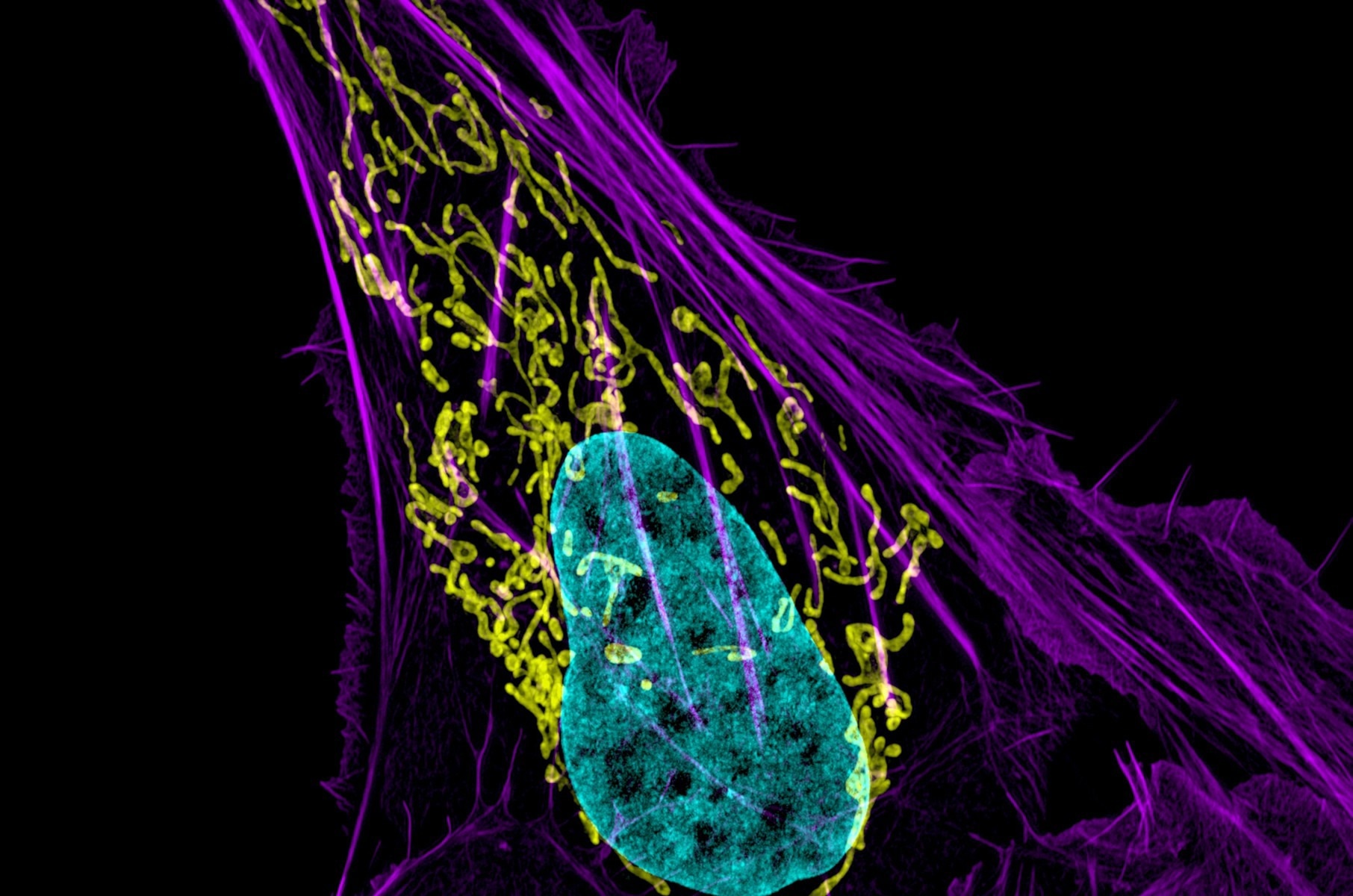 The study focused on disrupting the inner membrane of mitochondria, the primary producers of energy that fuels cell functions. Mitochondria are depicted in yellow in the image above of an osteocarcinoma cell. Image Credit: Dylan Burnette and Jennifer Lippincott-Schwartz, NICHD
The study focused on disrupting the inner membrane of mitochondria, the primary producers of energy that fuels cell functions. Mitochondria are depicted in yellow in the image above of an osteocarcinoma cell. Image Credit: Dylan Burnette and Jennifer Lippincott-Schwartz, NICHD
In a recent study, researchers used nanoparticles made specifically to target cancer cells to administer energy-disrupting gene therapy. According to experiments, the targeted therapy effectively reduces aggressive breast cancer tumors and glioblastoma brain tumors in mice.
Using a method that creates light-activated electrical currents inside the cell, the study team overcame a major obstacle to disassembling structures inside these cellular energy centers, known as mitochondria. The technology was dubbed mLumiOpto.
We disrupt the membrane so mitochondria cannot work functionally to produce energy or work as a signaling hub. This causes programmed cell death followed by DNA damage – our investigations showed these two mechanisms are involved and kill the cancer cell. This is how the technology works by design.”
Dr. Lufang Zhou, Study Co-Lead Author and Professor, Ohio State University
Co-lead author X. Margaret Liu, an Ohio State Professor of Chemical and Biomolecular Engineering, worked with Zhou on the study. Liu created the particles that were utilized to precisely deliver the gene therapy to cancer cells. Liu and Zhou both work as investigators at the Comprehensive Cancer Center at Ohio State University.
The study was published in the journal Cancer Research.
Since they are the main sources of energy that power cell processes, mitochondria have long been viewed as a desirable target for anti-cancer treatments; nevertheless, these attempts are hampered by their impermeable inner membrane.
Five years ago, Zhou’s lab unlocked the code by figuring out how to take advantage of the inner membrane’s weakness, which is an electrical charge differential that maintains the membrane’s structure and allows it to work as intended.
Zhou added, “Previous attempts to use a pharmaceutical reagent against mitochondria targeted specific pathways of activity in cancer cells. Our approach targets mitochondria directly, using external genes to activate a process that kills cells. That is an advantage, and we have shown we can get a very good result in killing different types of cancer cells.”
Zhou's previous cell studies demonstrated that a protein that generates electrical currents could damage the inner membrane of the mitochondria. Researchers used a laser to activate the light-induced protein. The team’s creation of an internal light source in this new project is crucial to converting the technology for clinical application.
The method entails transferring genetic information for two different kinds of molecules: an enzyme that emits bioluminescence and a light-sensitive protein called CoChR that can generate positively charged currents.
The proteins, which are created when their genes are expressed in mitochondria, are transported to cancer cells inside a modified viral particle. A subsequent injection of a certain chemical activates CoChR by turning on the enzyme’s light, which causes mitochondrial collapse.
Making sure that this treatment does not affect healthy cells is the other half of the fight.
Liu's lab focuses on developing targeted anti-cancer therapies. The well-characterized adeno-associated virus (AAV), a minimally infectious virus designed to carry genes and encourage their expression for therapeutic purposes, serves as the basis for the delivery mechanism used in this study.
By including a promoter protein to increase the production of the CoChR and bioluminescent enzyme exclusively in cancer cells, the scientists improved the system’s cancer specificity. Additionally, the researchers used human cells to create the AAV, which contained the gene-packed virus within a naturally occurring nanocarrier that resembled the extracellular vesicles found in biological fluids and human blood.
This construction assures stability in the human body because this particle comes from a human cell line.”
X. Margaret Liu, Study Co-Lead Author and Professor, Ohio State University
Lastly, the researchers created a monoclonal antibody that is intended to find receptors on the surface of cancer cells and connect it to the delivery particle.
Liu added, “This monoclonal antibody can identify a specific receptor, so it finds cancer cells and delivers our therapeutic genes. We used multiple tools to confirm this effect. After constructing AAVs with a cancer-specific promoter and a cancer-targeting nanoparticle, we found this therapy is very powerful to treat multiple cancers.”
Experiments conducted on mouse models revealed that the gene therapy approach dramatically decreased the tumor burden in two rapidly spreading, difficult-to-treat cancers: triple-negative breast cancer and glioblastoma brain cancer when compared to untreated mice. The treatment increased the survival of mice with glioblastomas and reduced the size of the tumors.
Studies using animal imaging also demonstrated that the effects of the gene therapy were only visible in cancerous cells and not in healthy tissue. Results also indicated that triggering an immune response against cancer cells in the tumor microenvironment was an additional advantage of attaching the monoclonal antibody.
The group is researching mLumiOpto’s further possible therapeutic benefits in triple-negative breast cancer, glioblastoma, and other cancers. Ohio State has filed a provisional patent application for the technologies.
Source:
Journal reference:
Chen, K., et. al. (2024) mLumiOpto Is a Mitochondrial-Targeted Gene Therapy for Treating Cancer. Cancer Research. doi.org/10.1158/0008-5472.CAN-24-0984