Accurate analysis of cannabinoid content in commercially available cannabis flower and fortified products is vital – particularly in products such as foods.
Liquid chromatography is an ideal tool when looking to confirm that product content descriptions and labels are accurate and truthful.
The use of cannabis products continues to increase as more states legalize recreational use of cannabis. Thorough, robust quantification of a range of cannabinoid compounds is therefore increasingly important, as failure to accurately confirm and report on the products’ contents can result in negative health impacts and a loss of consumer confidence.
Recently conducted studies of edible cannabis products obtained from licensed dispensaries in the state of California discovered that just 17% of 75 edible products purchased had been accurately labeled. The remaining products were either under-labeled (23%) or over-labeled (60%) in terms of their THC content.1
This article outlines a rapid, straightforward chromatographic method suitable for the analysis of 16 commonly analyzed cannabinoid structures (Figure 1).
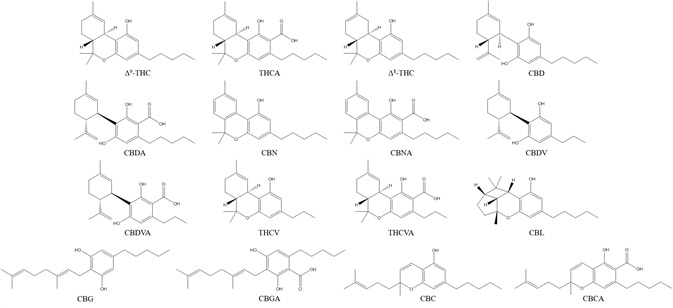
Figure 1. Chemical structures of the sixteen cannabinoids analyzed in this study. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Experimental
Hardware and software
A PerkinElmer LC 300 HPLC system was used to achieve chromatographic separation. This system was comprised of an LC 300 10k psi pump and an LC 300 autosampler fitted with an integrated column oven.
An LC 300 Photodiode Array (PDA) detector was used for detection, while instrument control, analysis and data processing were all conducted via the Simplicity™ Chrom CDS software platform.
Method parameters
Table 1 displays the LC parameters.
Table 1. LC Parameters. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| . |
. |
| Column |
PerkinElmer Quasar™ SPP C18, 2.6 μm, 150x3.0 mm (Part# N9308913) |
| Mobile Phase |
Solvent A: Water with 0.1% formic acid and 8 mM ammonium formate
Solvent B: Acetonitrile with 0.1% formic acid
Solvent Program: Isocratic
| Step |
Time (min) |
Flow Rate (mL/min) |
%A |
%B |
| 1 |
0.0 |
1.0 |
29 |
71 |
| 2 |
8.0 |
1.0 |
29 |
71 |
|
| Analysis Time |
8 min; Equilibration Time: 0.5 min |
| Pressure |
4300 psi/300 bar maximum |
Oven
Temperature |
40 ºC |
Sample
Temperature |
Ambient |
| Injection Volume |
10 μL (Partial Loop) |
| Analytical Wavelength |
228 nm Bandwidth: 5 nm Reference
Wavelength: 380 nm Bandwidth: 5 nm |
Data Collection
Rate |
5 pts/sec (Hz) |
| PDA Flowcell |
10 mm (standard) |
Solvents and standards
Every solvent utilized in this method was HPLC grade, with standard dilutions prepared using 80:20 methanol/water unless otherwise specified.
All sixteen 1 mg/mL cannabinoid standards were acquired from Cerilliant® (Round Rock, TX). These included:
- Δ9-tetrahydrocannabinol (Δ9-THC)
- Δ9-tetrahydrocannabinolic acid (THCA)
- Δ8-tetrahydrocannabinol (Δ8-THC)
- Cannabidiol (CBD)
- Cannabidiolic acid (CBDA)
- Cannabinol (CBN)
- Cannbinolic acid (CBNA)
- Cannabigerol (CBG)
- Cannabigerolic acid (CBGA)
- Cannabichromene (CBC)
- Cannabichromenic acid (CBCA)
- Cannabidivarin (CBDV)
- Cannabidivarinic acid (CBDVA)
- Tetrahydrocannabivarin (THCV)
- Tetrahydrocannabivarinic acid (THCVA)
- Cannabicyclol (CBL)
The method requires a 50 μg/mL stock standard mix solution. This was prepared by pipetting 1 mL of each standard into a 20 mL volumetric flask, filling this to the mark with water. This stock standard mix was also employed as the Cal-L6 calibration standard.
Further calibrants were prepared by serially diluting the standard mix to concentration levels of 25, 10, 5, 1 and 0.4 µg/mL in order to provide a 6-level calibration set.
Results and discussion
Figure 2 displays the chromatogram of the 50 µg/mL standard. All 16 cannabinoids eluted in less than 7 minutes.
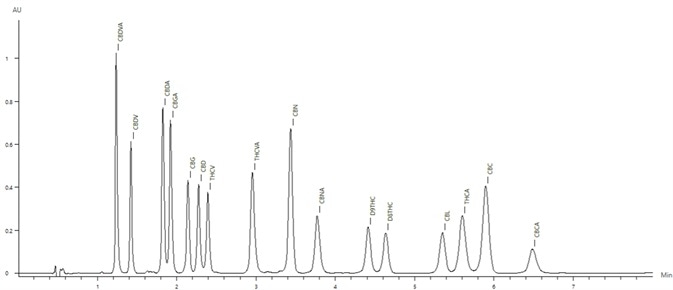
Figure 2. Chromatogram of the the 50 μg/mL cannabinoid standard. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Figure 3 displays the overlay of 10 replicate 50 µg/mL cannabinoid standard injections. These results demonstrate excellent reproducibility.
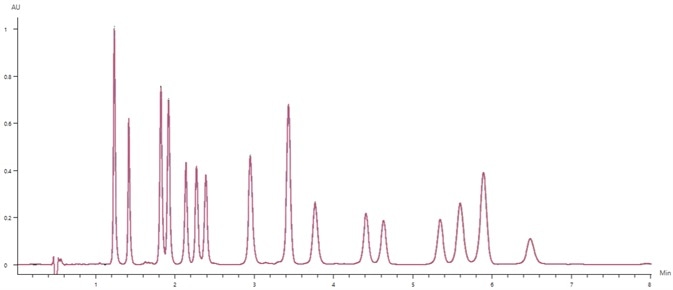
Figure 3. Chromatographic overlay of 10 replicates of the 50 μg/mL cannabinoid standard. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Analyzed cannabinoids were all found to possess a peak retention time precision (%RSD) of less than 0.16%, alongside a peak area precision of less than 0.7% (with the majority of analytes less than 0.5%).
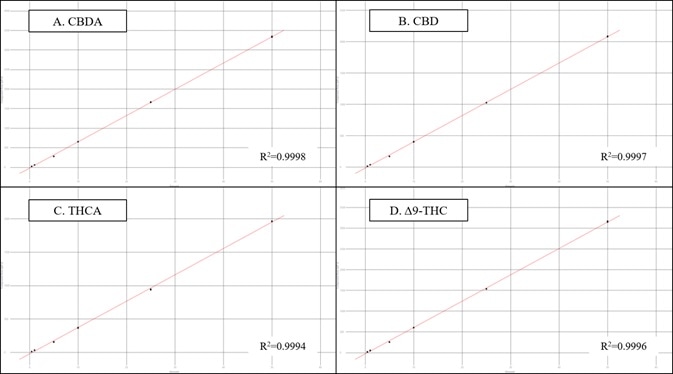
Figure 4. Results for the 6-level calibration sets for four example cannabinoids. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Figure 4 displays calibration results for four representative cannabinoids across a concentration range of 0.4 to 50 µg/mL. All 16 of the analyzed cannabinoids followed a linear (1st order) fit and exhibited R2 coefficients that were greater than 0.999 (n=3 at each level).
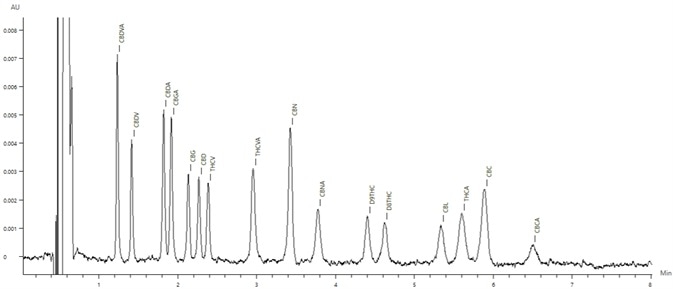
Figure 5. Chromatogram of the the 0.4 μg/mL cannabinoid standard. Image Credit: PerkinElmer Cannabis & Hemp Testing Solutions
Figure 5 displays the chromatogram of the low-level 0.4 µg/mL cannabinoid standard.
An 80:20 methanol/water solvent blank was injected after triplicate injections of the Cal-L6 (50 μg/mL) standard. No carryover was noted for any of the 16 analyzed cannabinoids.
LOQ (limit of quantitation) levels were determined for each analyte (Table 2) based upon the average response and signal-to-noise ratio for the Level 1 (0.4 µg/mL) calibration standard. These were run in triplicate.
Table 2. LOQs for the sixteen analytes, in order of elution. Source: PerkinElmer Cannabis & Hemp Testing Solutions
| Analyte |
Calculated LOD
(μg/mL; S/N ≥ 3) |
Calculated LOQ
(μg/mL; S/N ≥ 10) |
| CBDVA |
0.009 |
0.032 |
| CBDV |
0.016 |
0.053 |
| CBDA |
0.013 |
0.043 |
| CBGA |
0.014 |
0.045 |
| CBG |
0.022 |
0.074 |
| CBD |
0.023 |
0.078 |
| THCV |
0.025 |
0.082 |
| THCVA |
0.021 |
0.071 |
| CBN |
0.014 |
0.048 |
| CBNA |
0.037 |
0.124 |
| Δ9-THC |
0.042 |
0.141 |
| Δ8-THC |
0.048 |
0.161 |
| CBL |
0.051 |
0.170 |
| THCA |
0.041 |
0.138 |
| CBC |
0.025 |
0.083 |
| CBCA |
0.090 |
0.301 |
Conclusion
The examples presented here clearly demonstrate the capacity of a PerkinElmer LC 300 HPLC system with PDA detection to enable rapid, robust chromatographic separation and quantitation of a total of 16 commonly analyzed cannabinoids.
All results shown exhibited excellent retention time repeatability, alongside strong linearity over the tested concentration ranges. The method also provided LOQs of ≤ 0.15 μg/mL for almost all of the analytes investigated.
References
- Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-miller MO. Cannabinoid Dose and Label Accuracy in Edible Medical Cannabis Products. JAMA. 2015;313(24):2491-3.
- Cannabis oil vs hemp seed oil; Cannabis oil, CDB Oil, Medical Marijuana. https://cbd.org/
Acknowledgments
Produced from materials originally authored by Jamie Foss and Jason Weisenseel from PerkinElmer.
About PerkinElmer Cannabis & Hemp Testing Solutions
 With the cannabis and hemp markets continuing to grow rapidly and regulations strengthening, labs increasingly need streamlined access to best-in-class testing solutions geared toward the unique requirements of the industry. Whether your lab is well established or just starting up, PerkinElmer is a single-source vendor for instruments, methods, reagents, and consumables on hand to help enhance your testing capacity and get ahead of the competition.
With the cannabis and hemp markets continuing to grow rapidly and regulations strengthening, labs increasingly need streamlined access to best-in-class testing solutions geared toward the unique requirements of the industry. Whether your lab is well established or just starting up, PerkinElmer is a single-source vendor for instruments, methods, reagents, and consumables on hand to help enhance your testing capacity and get ahead of the competition.
They help drive analytical best practices and operating procedures and commit to ensuring your laboratory has maximum uptime. Learn about their various instruments, testing methods, and applications for cannabis analyses. Let them work with you to build an efficient workflow, so you can focus on growing your business.
Sponsored Content Policy: AZO Life Science publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of AZO Life Science, which is to educate and inform site visitors interested in medical research, science, medical devices, and treatments.