A great obstacle for pharmaceutical companies in the formulation of new drug molecules is drug solubility. It is essential to achieve the desired concentration of a drug in systemic circulation. Solubility determines the drug's bioavailability and is necessary to bring about the required pharmacological response.
Image Credit: Andy Shell/Shutterstock.com
Insoluble drugs have distinctly low bioavailability, however, almost 40% of all new chemical entities (NCEs) are insoluble. This is one of the biggest factors halting drug development.
Several methods for improving drug solubility have been proposed such as physical and chemical modifications. Some of these techniques are time-consuming and costly, preventing New Drug Entities (NCEs) from ever being released to the market.
Here, we discuss methods to achieve dissolution which may allow these drugs to reach their therapeutic potential.
Defining Solubility
Solubility is a property of a substance, determining the degree at which it can dissolve. A solute is dissolved into a solvent to form a homogenous solution where molecules are uniformly distributed.
The rate at which this occurs depends on chemical and physical parameters such as the solvent used, the polarity and particle size of the solute, the presence of other ions in the solvent, as well as temperature and pressure.
The opposing dissolution and precipitation forces occur in a dynamic equilibrium. A saturated solution is established when these two simultaneous processes occur at a continuous rate. This is when the maximum amount of solute is dissolved.
The importance of drug solubility
The most cost-effective and convenient method of drug delivery is by oral administration. However, the bioavailability of this dosage form depends notably on its solubility, amongst other parameters such as drug permeability, dissolution rate, metabolism, and efflux.
A drug must be aqueous at the site of absorption, in this case, the gastrointestinal mucosal lining, to reach the required plasma concentrations for pharmacological response. NCEs with high water solubility show good bioavailability; water-insoluble NCEs show slow absorption, inadequate bioavailability, and gastrointestinal mucosal toxicity.
A much higher dose is therefore required to reach therapeutic concentration levels in the plasma, posing safety concerns for patients. Optimizing the solubility of the dosage form would prevent this. Drug solubility is also a defining parameter in other dosage forms such as parenteral preparations.
The Biopharmaceutics Classification System differentiates drugs based on their solubility and intestinal permeability. It is used to predict its gastrointestinal absorption. Class II and IV drugs, both have solubility as the rate-limiting step in their bioavailability. When solubility is improved in these drugs, their bioavailability increases. It is an important factor for pharmaceutical companies to overcome, to improve drug therapeutic efficacy.
Methods for enhancing drug solubility
Several different methodologies exist for increasing the solubility of a drug. These range from physical to chemical modifications. Physical changes include particle size reduction, dispersion into carriers, and modifying the crystal state.
Chemical changes include surfactant use, salt formation, complexation, and adding hydrophilic groups. The selection of one of these techniques is dependent on drug characteristics, for example, the drug chemical structure, melting point, thermostability, and solubility. These methods can be used uniquely or in combination.
Surfactants
The oldest and simplest technique for enhancing drug solubility uses surfactants. They allow lipophilic molecules to dissolve in an aqueous solution by increasing surface tension. At defined concentrations, micellar solubilization occurs where the drug is encapsulated in a micelle.
Another common use is in the stabilization of drug suspensions. Surfactants may need to be used in combination with other techniques, due to their inability to sufficiently increase the solubility of highly insoluble drugs.
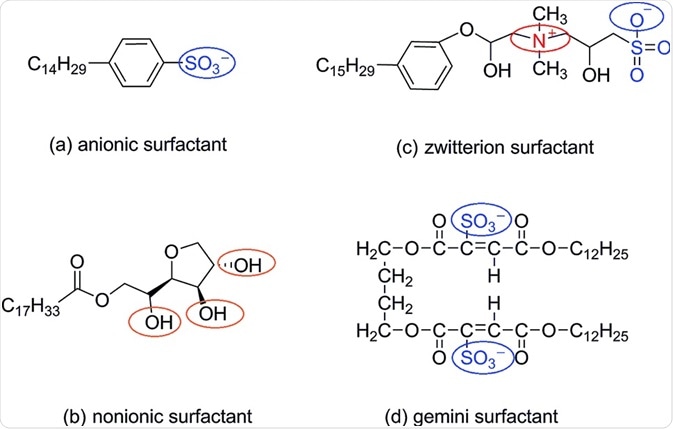
Image Credit: https://pubs.rsc.org/en/content/articlelanding/2019/ra/c8ra09670h#!divAbstract
Particle size reduction
Solubility is related to particle size: decreasing particle size increases its surface area to volume ratio, leading to greater interaction with the solvent and enhanced dissolution. Comminution and micronization are conventional techniques to reduce particle size.
Due to micronization only increasing the dissolution rate and not the equilibrium solubility, solubility might again not be increased sufficiently for highly insoluble drugs. Additionally, the mechanical and thermal stress induced by the milling process can lead to drug degradation. Regulatory concerns of comminution include the inability to control final product characteristics such as size and shape.
Solid Dispersion
This technique involves creating a solid product that contains at least 2 different components: a hydrophilic matrix and a hydrophobic drug. Hydrophilic carriers are added to drugs to increase their aqueous solubility. Examples of carriers include polyvinylpyrrolidone (PVP) and polyethylene (PEG).
There are several methods for achieving solid dispersion which include fusion (hot-melt), solvent evaporation, and hot-melt extrusion. A prerequisite for the hot-melt method is thermostability to avoid thermal decomposition of the drug.
Nanosuspension
Scaling drugs down to nanoparticles again reduces their particle size and increases their solubility. Nanosuspensions are colloidal dispersions of drug particles ranging between 200-600nm, stabilized by surfactants.
The advantage of this technique over others is that it can also be used on lipid insoluble drugs. Several methods can be used to achieve nanosuspension including precipitation, media milling, high-pressure homogenization, and combined precipitation and homogenization.
Supercritical fluid process
Particle size may also be reduced to nano sizes by the supercritical fluid process. Drugs are solubilized in a supercritical fluid which, with changes in pressure, can become highly compressible affecting its solvent potential. This technique enables the drug to be recrystallized at much smaller particle sizes with greater precision.
Inclusion complexes
The insertion of a non-polar region of a drug (guest) into the cavity of another (host) is an alternative method. Commonly used host proteins are cyclodextrins which have a hydrophobic interior for accommodating the guest region, and a hydrophilic surface allowing solubility of the molecule.
Crystal Engineering
Precisely designing molecular structures of drugs using intermolecular bonding to organize molecules is possible with crystal engineering. Drugs with improved solubility characteristics can be synthesized while maintaining their physical and chemical stability.
Sources
- Coltescu, A., Butnariu, M., and Sarac, I., 2020. The Importance of Solubility for New Drug Molecules. Biomedical and Pharmacology Journal, 13(02), pp.577-583.
- Sareen, S., Joseph, L., and Mathew, G., 2012. Improvement in solubility of poor water-soluble drugs by solid dispersion. International Journal of Pharmaceutical Investigation, 2(1), p.12.
- Savjani, K., Gajjar, A., and Savjani, J., 2012. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharmaceutics, 2012, pp.1-10.
Further Reading