Drug repurposing is vitally important to developing new therapeutic options for various diseases. The drug development process is lengthy, costly, and has a low success rate. Scientists repurpose drugs that may be used to treat another condition or may have failed to be successful in another indication to take advantage of the fact that the drug has already been through lengthy and costly clinical trials. They use information collected on the drug’s pharmacology to identify other diseases that the compound may be able to influence. Overall, drug repurposing is crucial as it reduces development costs and timelines.

Image Credit: paulista/Shutterstock.com
The benefits of repurposing drugs
Repurposing existing drugs is an attractive option is it reduces the risk, cost, and timeline of the drug development process. Drugs that have already been licensed for a therapeutic indication can be explored for potential efficacy in treating other illnesses.
Also, drugs that are developed for one indication but are deemed unsuitable due to side effects can still be of value as the unwanted side effects may be related to the drug’s pharmacology that may be valuable in the treatment of another disease.
Recent research conducted by researchers at LSE, the London School of Hygiene & Tropical Medicine, and KU Leuven in Belgium revealed that the average cost of bringing a new drug to market is $1.3 billion. Other recent evidence has deduced that the success rate of the drug development process is incredibly low, with only one in 20,000–30,000 compounds making it successfully through from compound selection to market approval.
Given the cost and success rate of drug discovery alongside its vital importance in improving human health, processes that speed up and reduce the cost of drug development are essential in helping effective drugs enter the market.
Leveraging off-target activity
When drugs display off-target activity (activity at more than one biological target), this is considered negatively as it produces unwanted side effects. However, this pharmacology is an indication that the drug may have other uses, and there is an opportunity to develop the drug as a therapy for a different disease.
Sometimes, a drug may be approved for a primary indication but later withdrawn for various reasons, including unwanted side effects, sub-optimal efficacy, or a new, more efficacious drug has entered the market. Repurposing these abandoned drugs takes advantage of the fact that they are compounds that have already been through the pre-clinical and clinical trials required to deem them safe for use in humans and to establish details on their pharmacology and optimal pharmacology dose.
Many widely used and well-known drugs are only available due to drug repurposing. Viagra, for example, was initially developed to treat angina but was repurposed to treat erectile dysfunction due to side effects observed in clinical trials. Byetta, a common drug used to treat weight loss, was initially developed to treat type 2 diabetes but was likewise repurposed due to unwanted side effects.
Another example is the drug thalidomide, which was initially developed to treat emesis but was ultimately withdrawn due to teratogenicity side effects; the drug is now used to treat multiple myeloma and leprosy.
The challenges of the COVID-19 pandemic and drug repurposing
The COVID-19 pandemic highlighted the importance of repurposing existing drugs. With the sudden emergence of a new disease impacting millions, it was essential to develop effective therapies rapidly. Usually, the drug development process takes years, a timeframe that wasn’t acceptable when considering the drugs were required to treat hundreds of thousands of patients and curb the pandemic.
Drug repurposing speeds up the traditional timeline of drug development. However, it is not without its challenges. The repurposing of drugs falls can be described as “off-label” use (OLU), where a physician prescribes a drug other than that which it has been approved for. While it is legal, regulatory drug agencies and scientific societies do not encourage it.
The problem with OLU is that side effects can be more severe and unpredictable, and the drug’s efficacy can be lower than required. However, getting a drug licensed for a new indication often requires significant investment.
Despite the challenges of drug repurposing, numerous drugs were successfully repurposed to treat COVID-19. Chloroquine and hydroxychloroquine were repurposed, as was tocilizumab, which was originally used as a recombinant humanized monoclonal antibody to treat rheumatoid arthritis and systemic juvenile idiopathic arthritis. Gilead’s antiviral remdesivir, initially indicated to treat a range of viruses, was the first drug to gain FDA approval to treat COVID-19 patients.
Anti-inflammatory drug dexamethasone, corticosteroid budesonide, and the antiparasitic drug ivermectin were also repurposed to treat COVID-19. The successful repurposing of these drugs highlights the value of the drug repurposing process, demonstrating its use in making new treatments available and fighting pandemic-proportioned outbreaks.
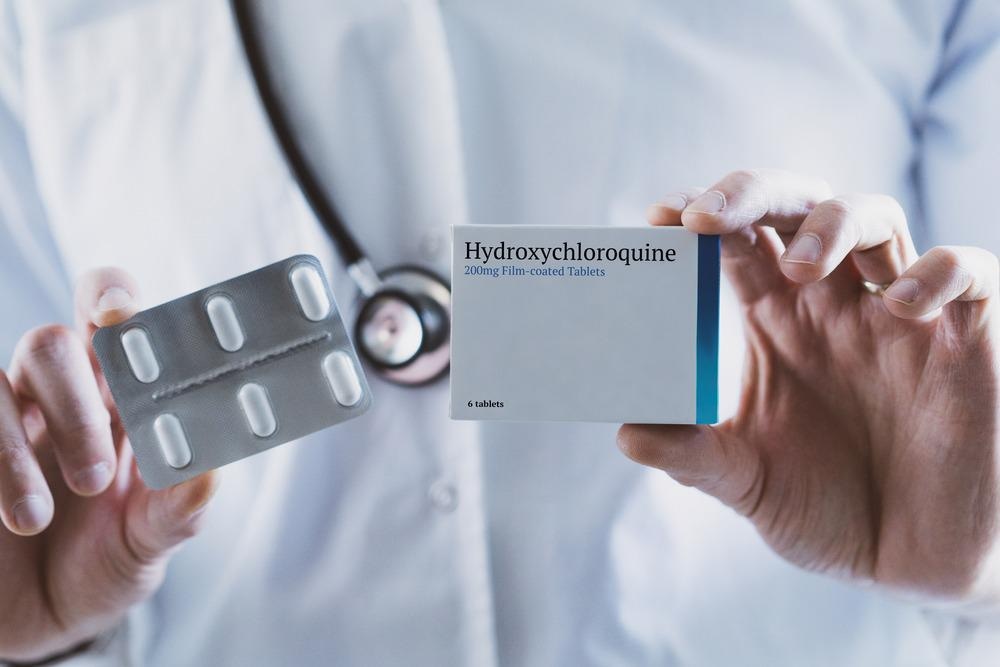
Image Credit: baranq/Shutterstock.com
Sources:
- Darcy Jimenez. 2021. No stone unturned: fighting Covid-19 with repurposed drugs. [Online]. Pharmaceutical Technology. Available at: www.pharmaceutical-technology.com/features/covid-19-repurposed-drugs/ Accessed March 2022
- Pushpakom, S., Iorio, F., Eyers, P., Escott, K., Hopper, S., Wells, A., Doig, A., Guilliams, T., Latimer, J., McNamee, C., Norris, A., Sanseau, P., Cavalla, D. and Pirmohamed, M., 2018. Drug repurposing: progress, challenges and recommendations. Nature Reviews Drug Discovery, 18(1), pp.41-58. https://www.nature.com/articles/nrd.2018.168
- Sultana, J., Crisafulli, S., Gabbay, F., Lynn, E., Shakir, S. and Trifirò, G., 2020. Challenges for Drug Repurposing in the COVID-19 Pandemic Era. Frontiers in Pharmacology, 11. https://www.frontiersin.org/articles/10.3389/fphar.2020.588654/full
Further Reading