Nanobodies are antibodies with a single variable domain located on a heavy chain, also known as VHH antibodies. Nanobodies are often seen as an alternative to conventional antibodies, and have significant differences in both production and use that influence their suitability.
What are Nanobodies?
The main difference between nanobodies and conventional antibodies has to do with their structure and their domains. Conventional antibodies have two variable domains, called VH and VL, which offer each other stability and binding specificity. Nanobodies have VHH domains and lack VL domains, but are still highly stable. Lacking the VL domain also means nanobodies have a hydrophilic side.
.jpg)
The camelid nanobody (center), first identified in camels, is a heavy-chain antibody that is much smaller and easier to program than antibodies found in most organisms, including humans, like that at left. At right, the monomeric camelid (red) is compared with the structure of the full-sized human antibody. VHH is a nanobody designed to target green fluorescent proteins used in proof-of-principle tests at Rice. Image Credit: Segatori Research Group/Rice University
With a whole host of advantages, the applications of nanobodies are highly varied. They are made from heavy-chain antibodies that occur naturally in camelids and sharks, and since their discovery 25 years ago have found use in a broad number of medical fields such as oncology, infection, and immunity. However, recent advances have seen nanobodies made to detect industrial chemicals in the environment, such as dyes or herbicides.
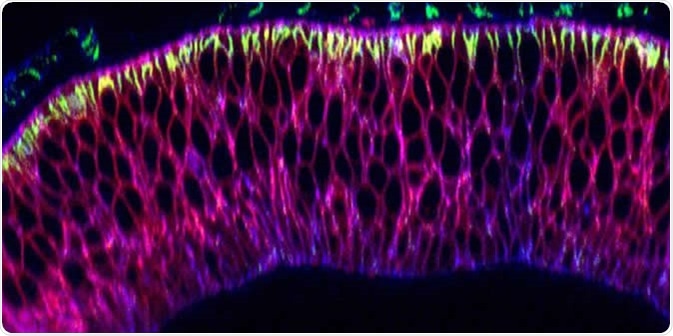
Nanobodies (pink) in the wing precursor of a fruit fly larva. Image Credit: University of Basel, Biozentrum
What are the Advantages of Nanobodies?
Nanobodies are often compared to poly- and monoclonal antibodies and antibody fragments, and the methods used to produce these. Nanobodies are easier to produce cheaply in bulk than polyclonal antibodies. Furthermore, they are stable in a wide range of temperatures, remaining functional at temperatures as high as 80°C. As an added bonus, the unfolding of the nanobody due to high temperatures has been shown to be fully reversible, unlike conventional antibody fragments. Nanobodies are also stable at extreme pH levels, able to survive exposure to gastric fluid.
Nanobodies are also compatible with genetic engineering methods, which allow scaffolding and alteration of amino acids to improve binding. Relating to structure, the hydrophilic side of nanobodies, that is not present in conventional antibodies, means they do not have issues with solubility and aggregation otherwise associated with conventional antibodies.
Monoclonal antibodies have been, and continue to be, useful for tumor therapy in several cancers. However, they tend to be very large, bearing four polypeptide chains, making their access to target tumors restricted. Nanobodies provide a smaller (1/10th the size of conventional antibodies), highly stable, alternative to antibodies
Nanobody production follows many of the same protocols as used in traditional antibody production. However, it also has distinct advantages not available with traditional antibodies, such as improved screening, improved isolation techniques, and no animal sacrifice.
Some of the differences between nanobodies and conventional antibodies are still being explored, but are believed to carry significant benefits. For example, it has been repeatedly established that conventional antibodies usually have flat surfaces. This means they do not bind well in grooves or cavities on the surface of the antigen. Nanobodies, on the other hand, preferentially bind clefts, including the active site cleft. The ability to bind active sites can have important implications for studying biological interactions but has not yet been well explored.
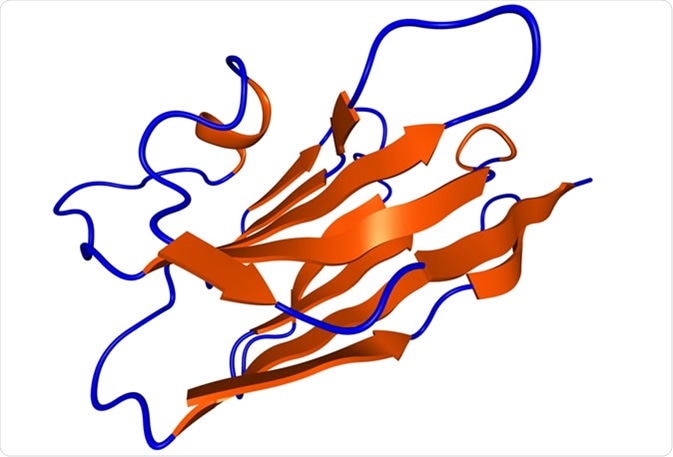
Nanobody protein therapeutic molecule. Nanobodies are small antibodies found in camels, dromedaries and llamas. - Illustration Credit: molekuul_be / Shutterstock.com
What are the Limitations of Nanobodies?
While relatively few, nanobodies still have significant limitations and disadvantages that limit their use. For one, the heavy chain antibodies from which nanobodies are developed can only be obtained from camelids and sharks. Traditional monoclonal antibodies, on the other hand, are obtained from mice. Therefore, the development of nanobodies requires larger, more complicated housing and animal husbandry for obtaining the desired antibody.
Monoclonal and polyclonal antibodies are slightly safer to produce than nanobodies, as there are biohazards involved in nanobody production not present for conventional antibody production. The biohazards result mainly from the use of hazardous bacteriophages for the selection of nanobodies. Other sources include plasmids, antibiotics, and recombinant DNA. These materials require safe disposal, which can be easily arranged depending on the institution, such as by autoclaves and disinfectants.
Further Reading
Last Updated: Feb 2, 2021