Cellular proteostasis is regulated by a crucial proteolytic machine called proteasome via selective degradation of ubiquitylated proteins.
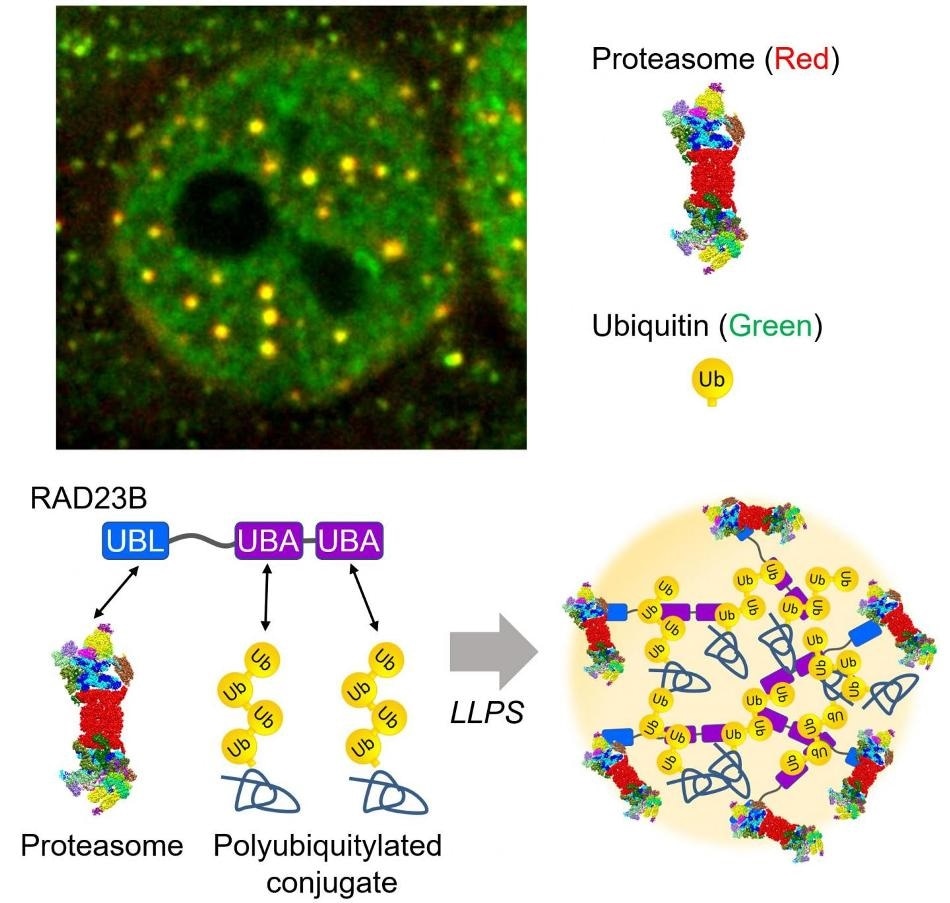
The proteasome droplets in the nucleus. Hyperosmotic stress induces liquid-liquid phase separation of the proteasome mediated by poly ubiquitinated proteins and RAD23B. Top: Fluorescent microscopic image of the proteasome droplets (Green: ubiquitin, Red: proteasome). Bottom: Schematic illustration of the proteasome droplets. Image Credit: Tokyo Metropolitan Institute of Medical Science.
Since protein homeostasis has to be maintained for human health, the breakdown of the ubiquitin-proteasome system (UPS) can lead to numerous diseases, like neurodegeneration, inflammation, and cancers. But the overall principles involved in the UPS are still unknown.
In the latest study, the scientists identified a new mode of protein degradation that was caused by the UPS in a stressed situation.
Around 2013, we discovered that proteasomes form nuclear foci in response to hyperosmotic stimuli. It was very impressive because the uniformly distributed proteasomes form foci in just a few seconds, but it took time to understand what this phenomenon means until we know liquid-liquid phase separation (LLPS).”
Yasushi Saeki, PhD, Study Lead Author, Tokyo Metropolitan Institute of Medical Science
Saeki continued, “LLPS is a rapid, reversible, and wide-spread compartmentalization mechanism in cells. The proteasome foci actually exhibit liquid-like behavior and a series of experiments revealed the LLPS of proteasomes is for degradation of ubiquitylated proteins.”
The results of the study were published in the Nature journal.
The proteasome droplets, induced by hyperosmotic stress, are a transient structure that vanishes within a few hours following sucrose treatment. These droplets also contain multiple proteasome-interacting proteins as well as ubiquitylated substrates.
While the formation of the proteasome droplets depends on the ubiquitylation of proteins, their disappearance depends on the proteasome’s activity, denoting that the proteasome droplets allow the degradation of proteins.
Both bucleolar stress and cell volume reduce in the presence of acute hyperosmotic stress, leading to the failure of ribosome biosynthesis and encouraging the buildup of orphan ribosomal proteins as a crucial UPS substrate in the nucleoplasm.
Undoubtedly, hyperosmotic stress promotes ubiquitylation and degradation of ribosomal proteins at the proteasome droplets; hence, the proteasome droplets are formed when there are increased levels of ubiquitylated proteins.
Along with his collaborators, Dr. Saeki further analyzed how the proteasomes are brought to this fluidic sub-compartment. The researchers eventually discovered a substrate shuttling factor of the proteasome, called RAD23B, as a vital molecule that causes the LLPS of ubiquitylated clients and the proteasome. One proteasome-binding domain (UBL) and two typical ubiquitin-binding domains (UBA) are present in RAD23B.
The researchers effectively reconstituted the ubiquitin-containing and the RAD23B-containing droplets in vitro and demonstrated that co-phase separation is driven by poor multivalent commutations between the long polymeric ubiquitin chains and the RAD23B UBA domains.
Collectively, the RAD23B acquires cellular ubiquitylated proteins through the UBA domains to develop droplets and subsequently recruits the proteasomes through the UBL domain.
This study is a good example of the interplay between ubiquitin signaling and LLPS. Given that ubiquitin mainly functions as a polymer, one of the biological meanings of the polymerization may be for LLPS. It will be greatly interesting to investigate whether other ubiquitin-binding proteins undergo phase separation.”
Yasushi Saeki, PhD, Study Lead Author, Tokyo Metropolitan Institute of Medical Science
“Also, it has been suggested that aggregation-prone proteins convert from liquid-like droplets to solid-like assemblies. In this context, acute hyperosmotic stress may risk irreversible accumulation of protein aggregates, especially when the proteasome activity is reduced,” Saeki concluded.
Source:
Journal reference:
Yasuda, S., et al. (2020) Stress- and ubiquitylation-dependent phase separation of the proteasome. Nature. doi.org/10.1038/s41586-020-1982-9.