Proteins can occasionally occur in fibrillar aggregates, known as amyloids, in the human body. Even though specific amyloids are found to have a biological function, the formation of amyloids is often linked to pathologies, like Parkinson’s and Alzheimer’s diseases.
High-speed atomic force microscopy enables visualizing and analyzing fibril formation of variants of a single protein. Image Credit: Kanazawa University.
Interpreting the exact formation of amyloid fibrils is important for gaining a deeper insight into the development of these disorders and for improving the treatment methods.
Using a method that helps visualize the growth over time, Takahiro Watanabe-Nakayama from Kanazawa University, Kenjiro Ono from Showa University, and collaborators have analyzed the formation process of certain amyloid fibrils.
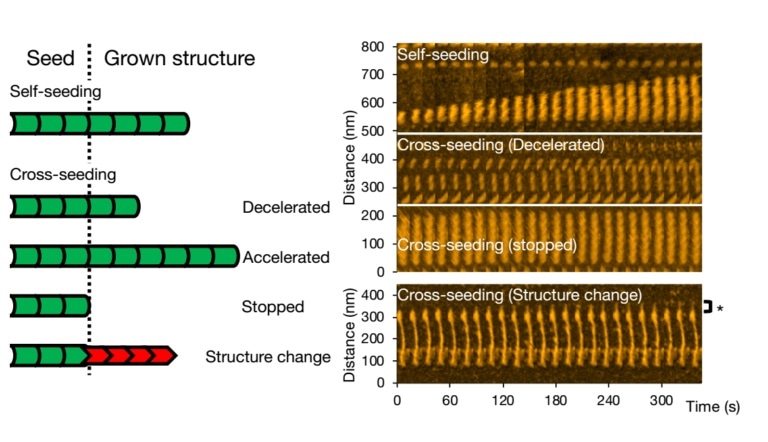
The team particularly examined the impact of mixing or cross-seeding different types of aggregate-forming proteins and identified changes in the fibril structures and elongation rates.
The scientists examined alpha-synuclein—a protein found abundantly in the human brain. Then, they observed what exactly happens when the wild-type alpha-synuclein molecules—the natural and most abundant variant—are allowed to form aggregates and also looked at how aggregation is different when mutant variants linked to Parkinson’s disease are introduced (cross-seeding).
Additionally, the researchers examined the effect of the pH level of the microenvironment where the growth of fibrils occurs.
Watanabe-Nakayama, Ono, and collaborators used high-speed atomic-force microscopy (HS-AFM) to record the aggregation of fibrils at high video rate and nanometer resolution for numerous cases.
Initially, the researchers observed the growth of single types of variants (self-seeding) and discovered that mutants created more numbers of aggregates, or that they aggregated more rapidly at neutral pH when compared to the wild-type variants. They also observed that elongation was much faster at a lower pH of 5.8, which is acidic, than at a higher pH of 7.4, which is basic.
Different kinds of scenarios can occur for cross-seeding. Fibril growth can be slowed down, expedited, or stopped altogether. It is possible to preserve the morphology of the original seed; however, it so happens that the structure of the ensuing fibril is different—standard structural forms are “spiral” or “straight.”
The team checked that the dynamics and structure of the fibrils as seen with the HS-AFM method correspond with the processes in the solution through fluorescence experiments; analogous conclusions were drawn.
The discoveries made by Watanabe-Nakayama, Ono, and collaborators are pertinent for gaining a deeper understanding of amyloid-related disorders.
Cross-seeding combined with variations in elongation rates has the effect of increasing the structural diversity of the resulting assemblies. This diversity may be reflected in distinct neurotoxic effects for various [protein] assemblies.”
Study Researchers, Kanazawa University
Source:
Journal reference:
Watanabe-Nakayama, T., et al. (2020) Self- and Cross-Seeding on α-Synuclein Fibril Growth Kinetics and Structure Observed by High-Speed Atomic Force Microscopy. ACS Nano. doi.org/10.1021/acsnano.0c03074.