Scientists from Kumamoto University, Japan have created an animal model that simulates cerebellar neurodegeneration and motor dysfunction analogous to that in spinocerebellar ataxia (SCA) by suppressing chaperone-mediated autophagy (CMA) in cerebellar neurons.
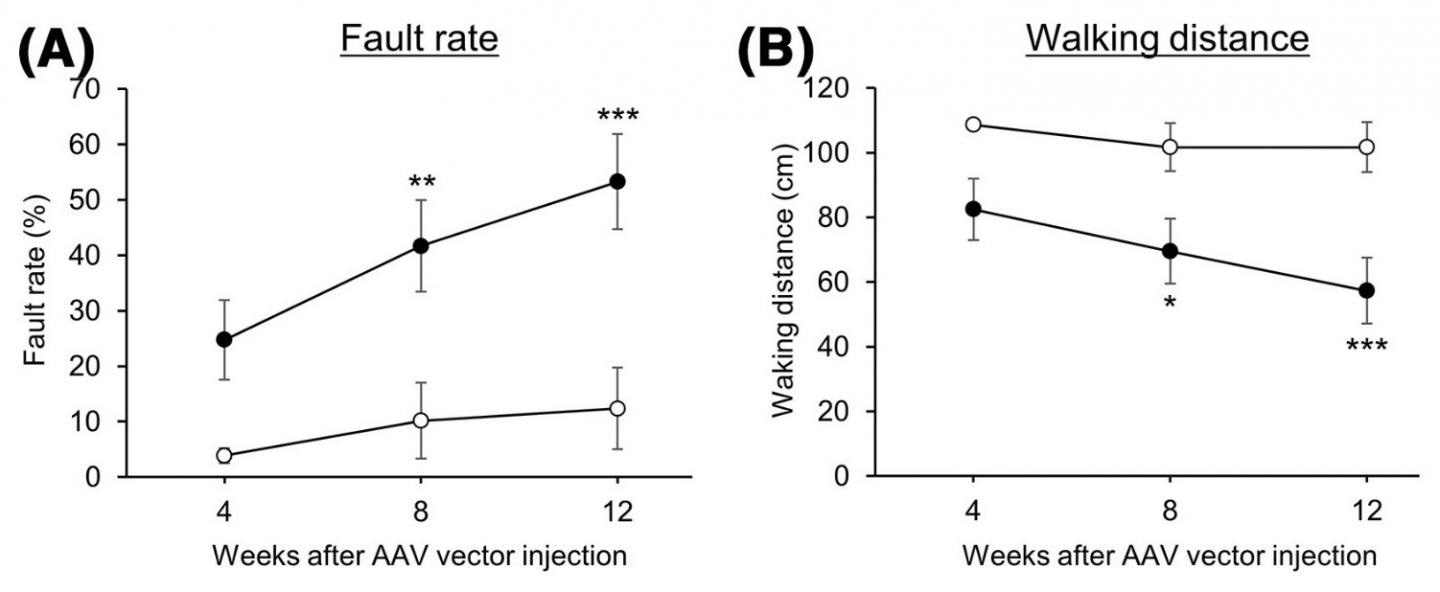
Motor function was evaluated in control mice (⚪) and mice with reduced CMA activity (⚫) by a beam walking test. A higher fault rate and shorter walking distances indicate lower motor function. Image Credit: Associate Professor Takahiro Seki.
Since CMA activity is decreased in cells that express SCA causing proteins, CMA is anticipated to turn into a novel therapeutic target for SCA—a disease that presently has no primary treatment.
SCA—an inherited, intractable neurological disorder—is caused by a number of genes. This disorder causes neurodegeneration and atrophy of the cerebellum and is distinguished by progressive motor dysfunction, like dysarthria (a brain disorder that leads to a loss of control the muscles used in speech) and staggering.
SCA is categorized into 48 different types in accordance with the causative genes, but the common mechanisms leading to ataxia cerebellar and atrophy in SCA are still unknown.
In living organisms, their cells have a mechanism known as autophagy that regulates homeostasis by breaking down intracellular components, like unnecessary proteins, and removing pathogenic microorganisms.
Chaperone-mediated autophagy (CMA) is a type of autophagy that carries intracellular components to lysosomes for degradation utilizing Hsc70 (a molecular chaperone) and LAMP2A (a lysosomal membrane protein).
Recent study has demonstrated that CMA plays a role in maintaining the homeostasis of neuronal proteins and that decreased CMA activity plays a role in the pathogenesis of Parkinson's disease. The association between CMA and neurodegenerative diseases, like Parkinson’s, has attracted a great deal of interest.
The Kumamoto University team created an original method for evaluating CMA activity in cells and studied its connection with the pathogenesis of neurodegenerative disorders. After identifying that decreased CMA activity was seen in cells expressing many SCA causing proteins, the team hypothesized that decreased CMA activity might be a standard part of the pathogenesis of SCA.
By injecting adeno-associated viral vectors, the researchers generated mice that had decreased CMA activity in cerebellar neurons. These vectors are capable of introducing a gene that particularly reduces LAMP2A into the mouse cerebellum which led to progressive motor dysfunction.
At early phases of motor dysfunction, histological analysis showed no effect on the morphology of cerebellar structure or cerebellar neurons.But glial cells, like microglia and astrocytes, became activated in the cerebellum. The activation of glial cells is a hallmark of neuroinflammation and leads to neurodegeneration in several neurodegenerative disorders.
At a significantly worse stage of motor dysfunction, the activation of glial cells and also the cerebellar neurodegeneration and related atrophy of the cerebellar cortex became prominent.
Such effects (progressive motor deficits and also neurodegeneration with associated atrophy of the cerebellar cortex)—seen in mice with decreased CMA activity in cerebellar neurons)—corresponds with the observations in SCA patients. In addition, numerous SCA mouse models have exhibited early glial cell activation.
Since CMA activity was found to be decreased by the proteins responsible for causing the SCA disease, the study results strongly indicate that decreased CMA activity in cerebellar neurons is a common molecular mechanism in the pathogenesis of SCA.
We have shown that CMA is a novel therapeutic target for the treatment of spinocerebellar ataxia and may lead to the development of a fundamental treatment that has not yet been established.”
Takahiro Seki, Study Lead and Associate Professor, Kumamoto University
Seki continued, “For SCA, the presence or absence of a causative gene can be determined by genetic diagnosis. However, even if a causative gene is found, there is no way to prevent the onset of the disease. If a safe compound that activates CMA can be developed, it could be a very effective treatment and preventive agent for SCA.”
Source:
Journal reference:
Sato, M., et al. (2020) Ataxic phenotype and neurodegeneration are triggered by the impairment of chaperone‐mediated autophagy in cerebellar neurons. Neuropathology and Applied Neurobiology. doi.org/10.1111/nan.12649.