Considering that phages are capable of destroying bacteria, they are of significant interest to science. Scientists from Berlin-based Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) are particularly interested in the tube employed by phages to insert their DNA into bacteria.
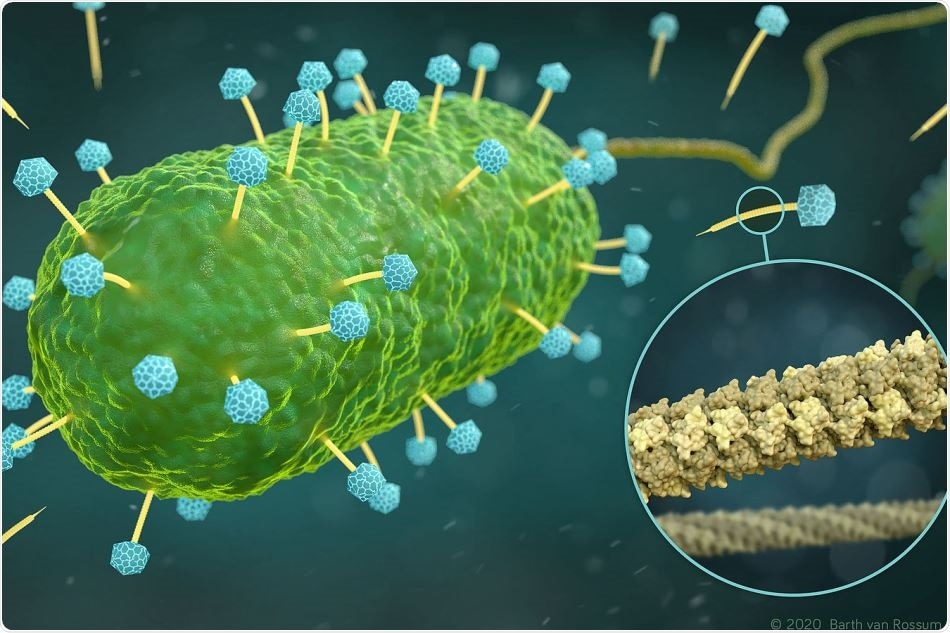
Artistic representation of phages of the family Siphoviridae (yellow and blue) that infect a bacterial cell (green). The excerpt (circle) shows the atomic structure of the DNA tube (yellow), through which the phages inject their DNA into the bacterium. Image Credit: Visualization: Barth van Rossum, Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP).
In association with collaborators from Forschungszentrum Jülich and Jena University Hospital, the team has now uncovered the three-dimensional (3D) structure of this major phage component in atomic resolution.
The researchers successfully achieved this by integrating a pair of techniques—that is, cryo-electron microscopy and solid-state NMR The study was recently published in the Nature Communications journal.
With rising resistance to antibiotics, phages have increasingly turned out to be the focus of studies. Phages are essentially viruses that occur naturally and have a highly useful trait—they insert their DNA into bacteria and multiply there until the bacterial cell is eventually killed. This is the reason why phages are also known as bacteriophages or bacteria eaters.
This strategy has already been demonstrated to combat multidrug-resistant bacteria. In 2019, the case of an English girl hit the headlines. The girl was suffering from a serious antibiotic-resistant infection and was treated with engineered phages.
But the extensive use of phage therapy still remains a long way off. A majority of the fundamental principles that are crucial for developing phage therapy are yet to be fully interpreted.
For instance, in the past, not much was known about the appearance of the actual design of the tube utilized by bacteriophages to insert their DNA into bacteria.
Researchers from the Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) in Berlin, along with collaborators from Forschungszentrum Jülich and Jena University Hospital, were now able to expose the 3D structure of this critical phage component in atomic resolution.
Designed for transporting DNA
“The structure and flexibility of the DNA tube attached to the icosahedron-shaped capsid are somewhat reminiscent of a spinal column,” said FMP’s Professor Adam Lange, elucidating one of the latest findings. “It seems to be perfectly designed for transporting DNA.”
The team was able to gain interesting insights into the function and structure of this advanced DNA transport pathway—in this example, from a phage SPP1 variant—by innovatively integrating cryo-electron microscopy (cryo-EM) with solid-state NMR.
The research team of Lange further advanced nuclear magnetic resonance spectroscopy (NMR), particularly for this job under an ERC Grant; Professor Gunnar Schröder, a cryo-EM expert from Forschungszentrum Jülich, conducted the electron-microscopic analyses.
The latest modeling algorithms were also needed for the computer-based integration of the two data sets to determine the structure. Professor Michael Habeck from Jena University Hospital developed these algorithms.
The key to success was combining the two methods, representing a methodological milestone.”
Adam Lange, Professor, Leibniz-Forschungsinstitut für Molekulare Pharmakologie
Although solid-state NMR is perfect for observing small details and flexible structures, the cryo-EM technique offers a better understanding of the overall architecture. The image, thus obtained, reveals that six gp17.1 proteins assemble into stacked rings, creating a hollow tube. The rings are joined by flexible linkers, rendering the tube extremely bendable.
We are now able to understand how negatively charged DNA is repelled from the likewise negatively charged interior wall of the flexible tube, passing through it smoothly. The bacteria are ultimately destroyed via this pathway.”
Maximilian Zinke, Study Lead Author, Leibniz-Forschungsinstitut für Molekulare Pharmakologie
The study was recently published in the Nature Communications journal.
Milestone for integrated structural biology
According to Adam Lange, the group leader, apart from representing a quantum leap forward in phage studies, the study will also develop “integrated structural biology”—the term denoting the integration of these two complementary techniques.
Due to the recent deployment of a novel high-resolution Titan Krios electron microscope, the infrastructure needed to realize this is currently available on Campus Berlin-Buch. A 1.2 gigahertz device will also be added to the existing NMR spectrometers shortly.
Equipped with cryo-EM and the most sensitive NMR spectrometer in the world, we will be very present in integrative structural biology in the future. This offers bright prospects for the campus and for the research location of Berlin.”
Adam Lange, Professor, Leibniz-Forschungsinstitut für Molekulare Pharmakologie
Source:
Journal reference:
Zinke, M., et al. (2020) Architecture of the flexible tail tube of bacteriophage SPP1.
Nature Communications. doi.org/10.1038/s41467-020-19611-1.