According to a new study performed by researchers from the Max Planck Institute for Evolutionary Biology in Plön, Germany, and the Chinese Academy of Sciences in Beijing, the possible genetic burden of mutations emerging from retrogenes is considerably greater than originally believed.
Retrogene function. Image Credit: MPI f. Evolutionary Biology.
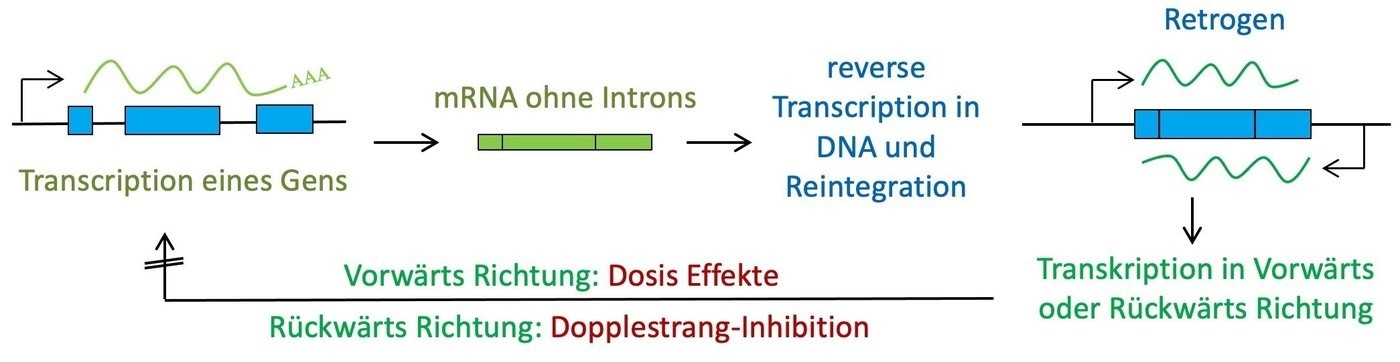
Genetic data is preserved in DNA and is transcribed as mRNA. Normally, mRNA is translated into proteins. But scientists have known for a long time that mRNA could also be transcribed back to DNA and re-inserted into the genome. These cases are known as retrogenes.
In a new article, researchers from the Max Planck Institute for Evolutionary Biology in Plön and the Zoological Institute of the Chinese Academy of Sciences in Beijing have reported that this process was earlier underestimated by a minimum factor of 1000, and that it is a significant new mutation mechanism.
There are two major explanations for that. Firstly, the common search algorithms employed in genome sequence analysis do not typically consider new insertions of retrogenes. Hence, these insertions continue to remain concealed in the large mass of data. And these insertions can only be systematically discovered using an optimized algorithm, such as the one designed by the researchers.
Secondly, the study authors demonstrated that a majority of the insertions are comparatively short-lived. Moreover, these insertions seem to be relatively rare in earlier genome comparisons between organisms.
Mutation by retrogenes is usually harmful
In the new research work, it was, therefore, important to explore populations that have developed only recently. The investigators observed that populations of mice that have been isolated for just around 3000 years bear different retrogenes, that is, in each population, retrogenes evolve at a very high rate but are gain lost again relatively rather quickly.
The reason for this is that retrogenes can be dangerous—even if they are embedded into non-coding DNA. If retrogenes are re-transcribed into mRNA (as is the case for the majority of them), this novel mRNA can adversely impact the mRNA of the gene from which they evolved. As a consequence, the retrogene functions as a regulatory mutant, which is generally negative.
The researchers have demonstrated that the genetic burden of this process is greater than that of point mutations, which so far have been the main focus of analyses. The team has therefore proposed that the retrogene mechanism should also be taken into account in the quest for disease-causing mutations.
Source:
Journal reference:
Zhang, W., et al. (2020) The mutational load in natural populations is significantly affected by high primary rates of retroposition. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2013043118.