Neurons cannot simulate their DNA and hence they work continuously to repair any damage caused to their genome.
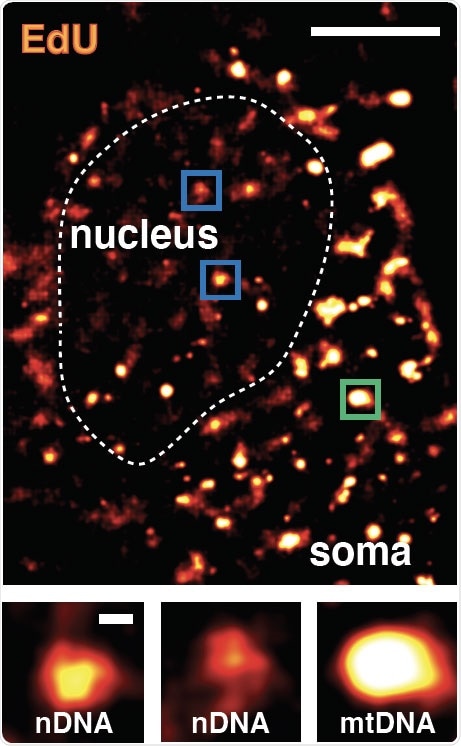
In this image of a neuron nucleus, bright spots show areas of focused genetic repair. Image Credit: Salk Institute/Waitt Advanced Biophotonics Center.
A new study performed by researchers from Salk Institute has now discovered that such repairs are not arbitrary but rather focus on guarding specific genetic “hot spots” that seem to play a crucial role in the identity and function of neurons.
The study results, published in the latest issue of Science journal on April 2nd, 2021, sheds new light on the genetic structures implicated in neurodegeneration and aging and may lead to the development of promising new treatments for various diseases, like Parkinson’s, Alzheimer’s, and other age-related dementia diseases.
This research shows for the first time that there are sections of genome that neurons prioritize when it comes to repair. We’re excited about the potential of these findings to change the way we view many age-related diseases of the nervous system and potentially explore DNA repair as a therapeutic approach.”
Rusty Gage, Professor and President, Salk Institute
Professor Gage is also the co-corresponding author of the study.
Neurons are different from other cells and they normally do not substitute themselves over time. This characteristic makes them one of the longest-surviving cells in the human body. The longevity of neurons also makes them much more significant, allowing them to repair lesions in their DNA as they get older and thus preserve their function over many years of a human life span.
As neurons become older, their potential to make these genetic repairs decreases, which may explain why individuals develop age-related neurodegenerative disorders, such as Parkinson’s and Alzheimer’s.
To find out how neurons preserve genome health, the researchers designed a novel method which they dubbed Repair-seq. They later created neurons from stem cells and injected them with synthetic nucleosides—that is, molecules that act as building blocks for DNA.
Such artificial nucleosides could be found across DNA sequencing and can be imaged. This clearly demonstrates where the neurons used these nucleosides to repair the DNA that was impaired by regular cellular processes.
Although the researchers hoped to see some prioritization, they were astounded by just how focused the neurons were when it comes to guarding specific parts of the genome.
What we saw was incredibly sharp, well-defined regions of repair; very focused areas that were substantially higher than background levels. The proteins that sit on these ‘hot spots’ are implicated in neurodegenerative disease, and the sites are also linked to aging.”
Dylan Reid, Study Co-First and Fellow, Vertex Pharmaceutics
Reid is also the co-corresponding author of the study and a former postdoctoral scholar at Salk Institute.
The researchers detected around 65,000 hot spots that enclosed about 2% of the neuronal genome. They subsequently applied proteomics techniques to identify which kinds of proteins were present at these hot spots, indicating several splicing-related proteins. (These play a role in the ultimate synthesis of other proteins.)
A majority of these locations seemed to be relatively stable when the cells were treated with agents that damage DNA, and the researchers also found that the most stable DNA repair hot spots were strongly linked to locations where chemical tags attach (methylation) that are best at estimating the age of neurons.
While prior studies have focused on detecting the DNA sections that undergo genetic damage, this is the first time where investigators have looked for certain sections of the genome that are being heavily repaired.
We flipped the paradigm from looking for damage to looking for repair, and that’s why we were able to find these hot spots. This is really new biology that might eventually change how we understand neurons in the nervous system, and the more we understand that, the more we can look to develop therapies addressing age-related diseases.”
Dylan Reid, Study Co-First and Fellow, Vertex Pharmaceutics
“Understanding which areas within the genome are vulnerable to damage is a very exciting topic for our lab. We think Repair-seq will be a powerful tool for research, and we continue to explore additional new methods to study genome integrity, particularly in relation to aging and disease,” concluded Gage, who also holds the Vi and John Adler Chair for Research on Age-Related Neurodegenerative Disease.
Source:
Journal reference:
Reid, D. A., et al. (2021) Incorporation of a nucleoside analog maps genome repair sites in postmitotic human neurons. Science. doi.org/10.1126/science.abb9032.