CRISPR-Cas9 was first discovered as a bacterial defense against invading viruses, but its extraordinary ability to modify specific areas of DNA has made it a favorite among gene-editing tools for researchers. The continued attempt to investigate the CRISPR-Cas9 system’s potential is ushering in further advances to this technology.
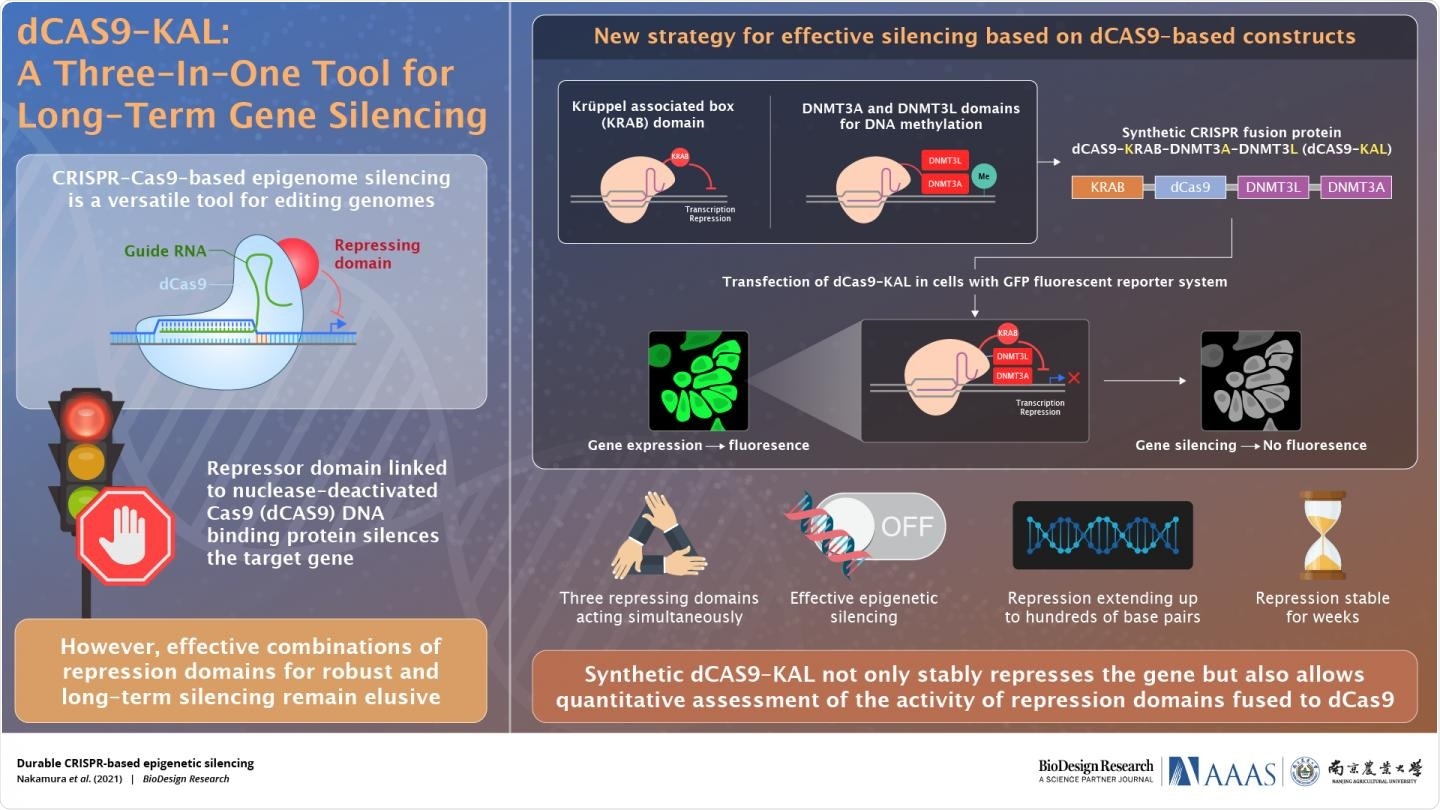
Synthetic dCAS9-KAL not only stably represses the gene but also allows quantitative assessment of the activity of repression domains fused to dCas9. Image Credit: BioDesign Research.
Scientists from Stanford University in the United States have devised a CRISPR-Cas9 system that produces very effective silence of target genes, as revealed in a paper published in BioDesign Research.
The adaptability of CRISPR-Cas9-based gene editing is largely due to Cas9 protein modification. The Cas9 protein’s endonuclease property is eliminated in this method, resulting in a deactivated Cas9, or dCas9. Many effector proteins, including a wide range of gene-altering enzymes, are subsequently fused to dCas9 for targeted DNA binding at specified places.
The dCas9 complex upregulates or downregulates the target gene depending on whether it is coupled with an activating or repressive transcription factor. However, the ability of such complexes to change genes is only temporary, as the effects last only as long as the regulatory proteins’ effector domains are physically bound or actively targeted to the region of interest.
A longer-lasting effect of silencing is desirable for practical objectives, such as inhibiting the action of a disease-causing mutation.
The Cas9 endonuclease, that stops the expression of a target gene, is analogous to a brake which stops the car by breaking its engine. In a modified dCas9-repressor fusion system, that contains a transcriptional repressor, the car remains stopped as long as one holds down the brake pedal actively. However, we aimed to develop a silencing system that, like a parking gear, prevents wheel movement until you switch the car out of it.”
Dr Lei S. Qi, Study Corresponding Author, Stanford University
To do this, the researchers used a strategy based on eukaryotic genome epigenetic regulation, in which gene expression is stably and reversibly altered by direct modification of genomic areas. Methylation of histone proteins and DNA is used in epigenetic silencing.
Dr. Qi and his colleagues combined the dCas9 protein with the KRAB (Krüppel-associated box) transcription repressor domain and the DNA methylating domains of DNMT3L and DNMT3A, two strong epigenetic modifiers.
They gave the construct the moniker dCas9-KAL and put it to test in a cell-based reporter system. The dCas9-KAL construct, which was engineered to localize at the promoter of the fluorescent protein EGFP, inhibited fluorescence for weeks when it was stably incorporated into human cells expressing the fluorescent protein EGFP.
The development of a stable and long-lasting epigenetic repressor has multiple consequences. Successful silencing of essential or disease-inducing genomic elements may improve therapy choices for cancer and other genetic diseases.
Furthermore, the scientists will be able to analyze the activity of various domains or combinations of domains fused to dCas9 using the unique construct-synthetic reporter system developed in this study.
Since its adoption, CRISPR-Cas9 has revolutionized the face of genetic modification. Our system, as a powerful addition to the CRISPR toolbox, will facilitate further research in the field. It can be used to better engineer cells with desired behaviors, which could find use in the development of custom cell types with wide-ranging research and therapeutic applications.”
Dr Muneaki Nakamura, Study Lead Author, Stanford University
The results of the researchers are an important addition to the CRISPR-Cas9 system, which is a molecular Swiss knife.
Source:
Journal reference:
Nakamura, M., et al. (2021) Durable CRISPR-Based Epigenetic Silencing. BioDesign Research. doi.org/10.34133/2021/9815820.