Scientists have created a new formulation based on regulatory T-cell exosomes (rEXS) to administer vascular endothelial growth factor (VEGF) antibodies for choroidal neovascularization therapy.
Regulatory T-cell exosome-based formulation to deliver VEGF antibodies for synergistic therapy in CNV mouse model. Image Credit: Yingtian Tian and Fan Zhang.
The breakthrough was achieved by scientists from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences, Beijing Chaoyang Hospital, and the University of Queensland. The study was reported on July 26th, 2021, in Nature Biomedical Engineering.
In general, ocular neovascularization is related to age-related macular degeneration, diabetic retinopathy, and other ocular diseases that can result in severe vision loss.
The existing clinical treatment for ocular neovascular disease involves intravitreal injection of VEGF antibodies (aV) to inhibit the activity of VEGF and block pathogenic angiogenesis. But this therapy alone faces the challenges of limited efficacy, fast metabolism with the aqueous humor, and poor accumulation in lesions. A vast proportion of patients still exhibit an incomplete response to the above aV treatment.
As part of their study, the researchers gathered aqueous humor samples from a huge cohort of patients and measured VEGF and other proinflammatory cytokines.
We observed a strong association between inflammation and high VEGF expression in aqueous humor samples.”
Yong Tao, Professor, Beijing Chaoyang Hospital
They thus suggested a synergistic therapeutic strategy that integrates anti-VEGF and anti-inflammatory therapies.
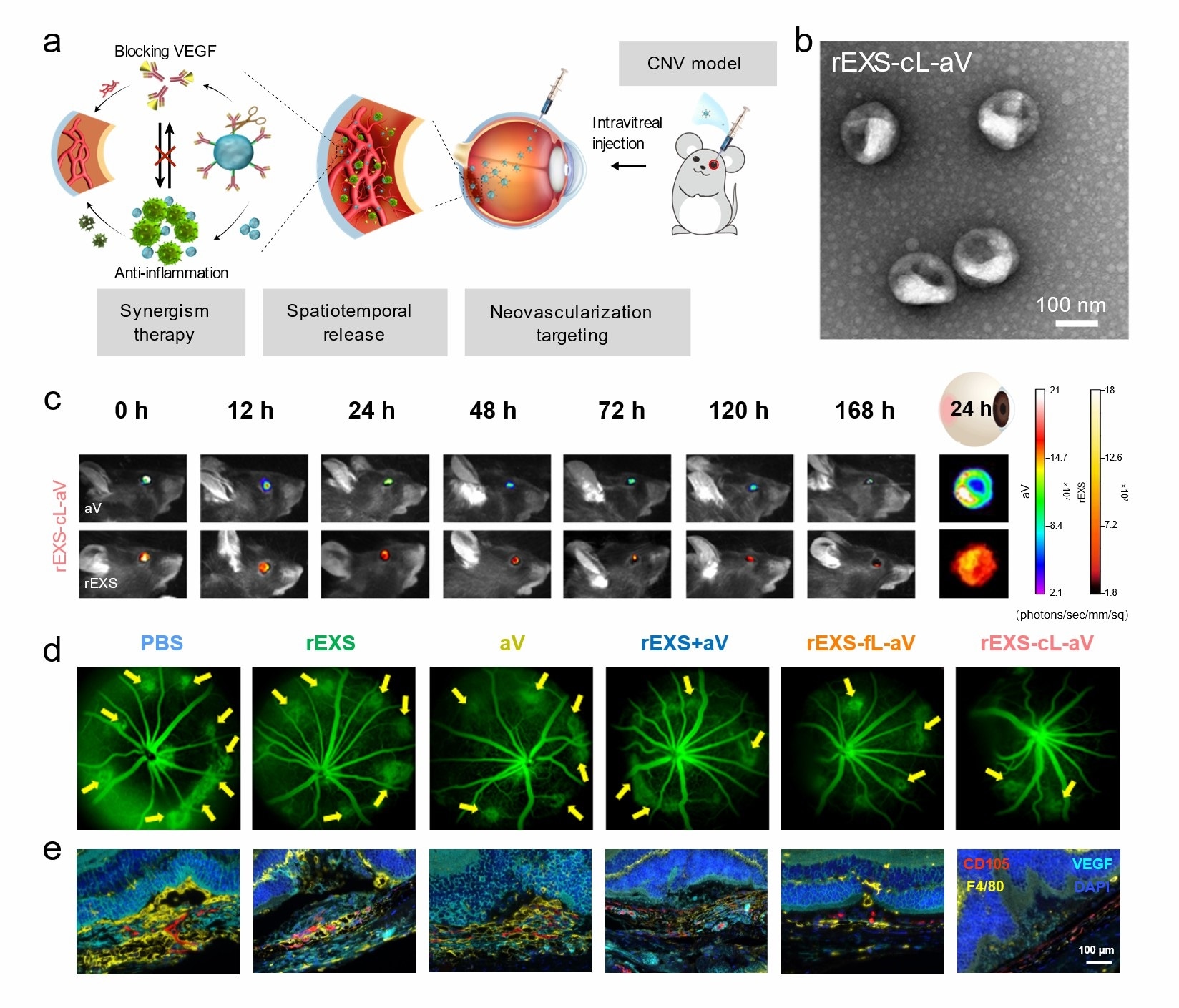
As part of the approach, exosomes derived from regulatory T-cells were used to conjugate aV using a peptide linker (cL), which underwent cleavage by matrix metalloproteinases (MMPs) in inflammatory lesions.
This design concept could achieve efficient spatiotemporal delivery for combination therapy. After intravitreal injection, rEXS-cL-aV exploited the ability of rEXS to localize in neovascularization lesions and, upon MMP-mediated cleavage, released rEXS and aV to suppress inflammation and VEGF activity, respectively.”
Wei Wei, Professor, Institute of Process Engineering, Chinese Academy of Sciences
The researchers confirmed the strong therapeutic efficacies in both murine and nonhuman primate models of choroidal neovascularization.
This study is still at the preclinical stage. Given that rEXS can be produced from the patients’ own cells and aV has been approved for clinical use, our rEXS-cL-aV has the potential for translation to clinic.”
Di Yu, Professor, University of Queensland
According to a peer reviewer from Nature Biomedical Engineering, “The technology is novel and the treatment efficacy is impressive.” The reviewer also reiterated that “collectively, the work provides a substantial technological and potentially therapeutic advance for the treatment of neovascular disease.”
Source:
Journal reference:
Tian, Y., et al. (2021) Reduction of choroidal neovascularization via cleavable VEGF antibodies conjugated to exosomes derived from regulatory T cells. Nature Biomedical Engineering. doi.org/10.1038/s41551-021-00764-3.