Scientists from Uppsala University recently documented and visualized genes linked to hearing loss in the human inner ear.
The research was a unique collaboration study between geneticists and otosurgeons. The observations reveal that distinct subcellular structures seen in the ear—the cochlea—are engaged in the differences in risks of age-related hearing loss among people. The research was published in the journal BMC Medicine.
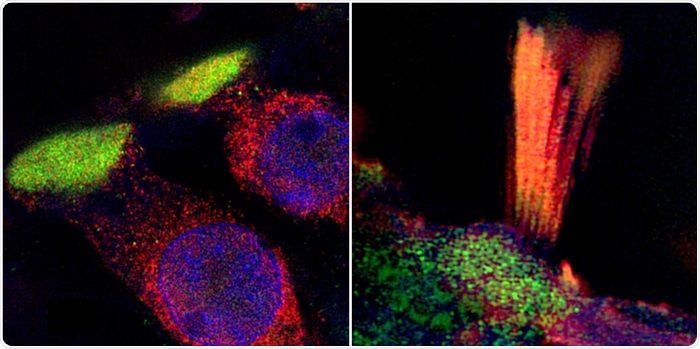
Super-resolution structured illumination microscopy (SR-SIM) show critical proteins in human hair cells associated with age-related hearing loss. (Left) LMO7 protein in the plate (green) that anchors the hair tuft to the cell (cilia). (Right) TRIOBP (green and yellow) is another essential protein expressed in the cilia. Image Credit: Dr Wei Liu.
Hearing loss is a potentially weakening condition that affects over 1.23 billion individuals globally. The commonly observed form of hearing loss—representing 90% of all cases—is connected to the deteriorating effects of aging on hearing, i.e., presbycusis or age-related hearing loss. But the molecular mechanisms underlying the progress of age-related hearing loss and individual differences in risk are less elucidated.
The recent research, a unique collaboration between geneticists and otologists at Uppsala University, enabled functional monitoring analysis of candidate genes from genome-wide association studies (GWAS) utilizing immunohistochemistry in the cochlea of humans.
The cochlea, and in particular the hearing organ, the organ of Corti, is a highly vulnerable structure that is difficult to analyze since it is surrounded by the hardest bone in the body. We have been able to study some of the molecular components of human hearing that are critical for the conversion of sound to nerve electric impulses.”
Helge Rask-Andersen MD, Senior Professor, Department of Surgical Sciences, Uppsala University
Genetic variants at 67 genomic regions contributed to the enhanced risk of age-related hearing loss. Genome-wide association studies (GWAS) on hearing-related traits were carried out in the UK Biobank, which had half a million participants from the United Kingdom. Genetic associations are hard to decipher by themselves and monitoring experiments are generally needed before causal genes can be inferred.
It is an amazing opportunity to be able to follow up our findings in human cochlear samples since there are molecular differences between the hearing organ of humans and other mammals.”
Mathias Rask-Andersen, Associate Professor, Department of Immunology, Genetics and Pathology, Uppsala University
Immunofluorescent antibodies and super-resolution structured illumination microscopy (SR-SIM) were used to visualize candidate proteins from GWAS by Dr. Wei Liu, MD, and Associate Professor at the Department of Surgical Sciences. Numerous proteins were seen within the spiral ganglion, containing the neuronal cell bodies that innervate the hair cells in the organ of Corti and transport neuronal impulses to the brain through the cochlear nerve.
The scientists also visualized hearing loss-linked proteins in discrete subcellular domains in the hair cells for the first time in humans; similar to TRIO and F-actin-binding protein (TRIOBP) in the hair tufts (stereocilia) and LIM domain only protein 7 (LMO7) in the cuticular plate—an actin-rich structure that anchors stereocilia to the cell body.
The stereocilia are the microscopic or nano-sized ‘hairs’ that project from the hair cells of the organ of Corti. They respond to mechanical vibrations from sounds that reach humans and are transferred and amplified from the eardrum to the inner ear by the small middle ear bones.
The observations from the recent research reveal that common genetic variations linked with age-related hearing loss impact the structures of the cochlea, specifically the neuronal mechanisms of the spiral ganglion, and also structures involved directly in the transduction of mechanical stimuli to neuronal impulses.
This understanding might help better comprehend the biological processes that lead to age-related hearing loss and create strategies for prevention like novel pharmacological treatments.
Source:
Journal reference:
Liu, W., et al. (2021) A combined genome-wide association and molecular study of age-related hearing loss in H. sapiens. BMC Medicine. doi.org/10.1186/s12916-021-02169-0.