Similar to how images of cats can be developed utilizing artificial intelligence, novel proteins too can be now made with similar tools. A group including scientists from the University of Washington, Rensselaer Polytechnic Institute, and Harvard University detail the development of a neural network that “hallucinates” proteins with a novel, stable structures. The study was published in the journal Nature.
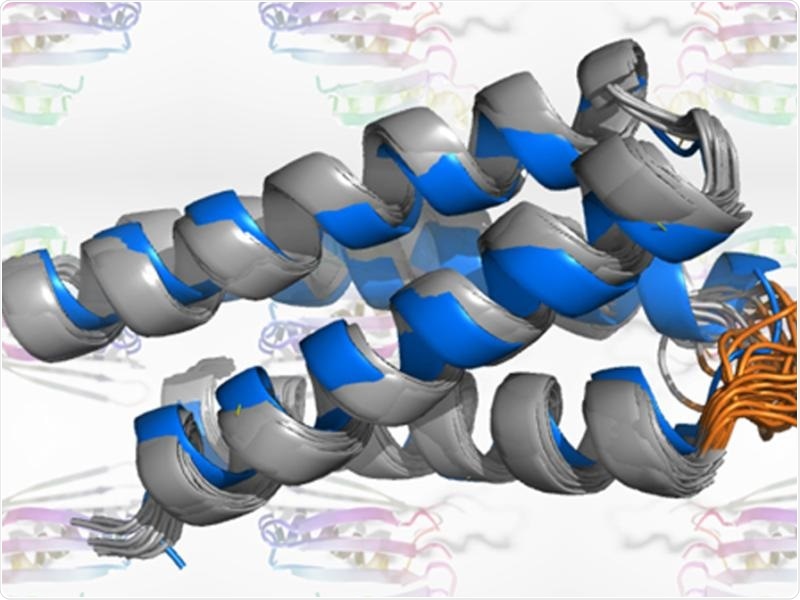
A neural network “hallucinated” proteins that were synthesized to confirm their structure. Image Credit: Rensselaer Polytechnic Institute.
The potential to hallucinate brand-new proteins that bind particular biomolecules or form desired enzymatic active sites is very exciting.”
Gaetano Montelione, Professor, Chemistry and Chemical Biology, Rensselaer Polytechnic Institute
The synthesized versions of “hallucinated” proteins were fabricated by a neural network were examined.
Proteins are string-like molecules seen in each cell that instinctively fold into complex three-dimensional shapes. These folded shapes are vital for each process in biology, including DNA repair, cellular development, and metabolism. However, the complexity of protein shapes makes it hard to analyze.
Biochemists mostly use computers to foretell how protein sequences or strings would fold. Recently, artificial intelligence techniques such as deep learning and neural networks revolutionized the precision of this work.
For this project, we made up completely random protein sequences and introduced mutations into them until our neural network predicted that they would fold into stable structures. At no point did we guide the software toward a particular outcome—these new proteins are just what a computer dreams up.”
Ivan Anishchenko, Study Co-Lead Author and Postdoctoral Scholar, Institute for Protein Design, University of Washington School of Medicine
Ivan Anishchenko is a postdoctoral scholar at the Baker laboratory.
The researchers anticipate that in the future it should be possible to lead artificial intelligence so that it creates novel proteins with beneficial features.
“We’d like to use deep learning to design proteins with function, including protein-based drugs, enzymes, you name it,” remarked co-lead author Sam Pellock, a postdoctoral scholar in the Baker laboratory.
The scientists produced 2,000 new protein sequences that were expected to fold. More than 100 of these were generated in the lab and analyzed. A comprehensive analysis of such three proteins affirmed that the shapes predicted by the computer were recognized in the laboratory.
Our solution NMR studies, along with X-ray crystal structures determined by the University of Washington team, demonstrate the remarkable accuracy of protein designs created by the hallucination approach.”
Theresa Ramelot, Study Co-Author and Senior Research Scientist, Rensselaer Center for Biotechnology and Interdisciplinary Studies
Theresa Ramelot works in the Montelione laboratory.
“The hallucination approach builds on earlier observations we made together with the Baker lab revealing that protein structure prediction with deep learning can be quite accurate even for a single protein sequence, without recourse to contact predictions usually obtained by analysis of many evolutionary-related protein sequences,” states Montelione.
According to senior author David Baker, recipient of the 2021 Breakthrough Prize in Life Sciences, “This approach greatly simplifies protein design.” He further adds, “Before, to create a new protein with a particular shape, people first carefully studied related structures in nature to come up with a set of rules that were then applied in the design process. New sets of rules were needed for each new type of fold.”
“Here, by using a deep-learning network that already captures general principles of protein structure, we eliminate the need for fold-specific rules and open up the possibility of focusing on just the functional parts of a protein directly,” stated Baker.
“Exploring how to best use this strategy for specific applications is now an active area of research, and this is where I expect the next breakthroughs,” concluded Baker.
Source:
Journal reference:
Anishchenko, I., et al. (2021) De novo protein design by deep network hallucination. Nature. doi.org/10.1038/s41586-021-04184-w.