Researchers from the University of California, Berkeley, have published a study that provides the groundwork for novel approaches to pathogen evolution. This study was published in the journal Molecular Plant-Microbe Interactions.
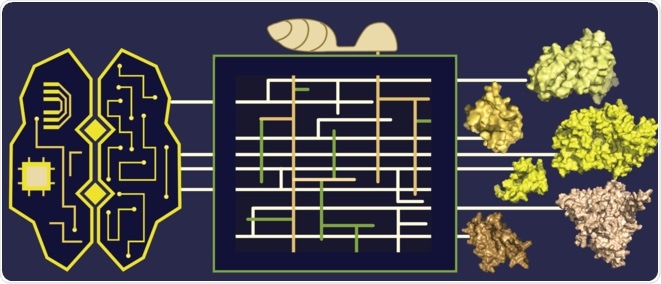
Elucidating the structures of phytopathogens’ secreted proteins with machine learning-based structure prediction tools. Machine learning and plant-pathogen interaction commonly have a black box. During the prediction from the input primary sequences to protein structures, we don’t exactly know what happens. Similarly, we do not fully understand the complex interaction at the interface of plants and pathogens. The box in the middle captures the complexity within this black box. Image Credit: Kyungyong Seong and Ksenia V. Krasileva
Our research highlights that template-free modeling that uses machine learning is indeed superior to template-based modeling for the secreted proteins of the destructive fungal pathogen Magnaporthe oryzae.”
Kyungyong Seong, Study First Author, Department of Plant and Microbial Biology, University of California
Pathogens employ virulence factors called effectors, which are critical for their survival. One of the most extensively used approaches is homology modeling; however, this needs the usage of templates of solved effector structures and solving all of the effector structures is too difficult. There are several effector proteins encoded in pathogens’ genomes for each structure to be solved experimentally.
Seong and his colleague Ksenia V. Krasileva utilized a novel structure prediction approach that can model 500 secreted proteins that the template-based method had previously failed to predict.
About 70% out of the 1,854 secreted proteins were modeled in our study, and their structures provide an extra layer of information about the effectors based on their similarity to each other or other solved protein structures. We demonstrate that new structure prediction methods apply well to the problem of deciphering pathogen virulence factors and other secreted proteins that often have little sequence similarity among themselves or to other proteins.”
Ksenia V. Krasileva, Study Corresponding Author, Department of Plant and Microbial Biology, University of California
Scientists can now map thousands of secreted proteins and discover missing evolutionary connections between them using this new technology. “We believe our research was the first to apply the concept of structural genomics on a plant pathogen in the new era of machine-learning structure prediction,” explained Seong.
As the accuracy of structure prediction improves further, it will become more common to see articles that incorporate large-scale protein structure prediction data. Our article may spark some ideas of how to use such data, leading some scientists to explore opportunities ahead of other.”
Ksenia V. Krasileva, Study Corresponding Author, Department of Plant and Microbial Biology, University of California
They also discovered that M. oryzae has a large number of unique sequence-unrelated structurally comparable effectors, as well as structurally similar effectors in other phytopathogens. This shows that pathogens attack plants through a group of effectors that originated in the same place but have mainly diverged in sequences over time to infect plants.
Source:
Journal reference:
Kyungyong S. & Krasileva, V. K. (2021) Computational Structural Genomics Unravels Common Folds and Novel Families in the Secretome of Fungal Phytopathogen Magnaporthe oryzae. Molecular Plant-Microbe Interactions. doi.org/10.1094/MPMI-03-21-0071-R.