The process of making proteins from genes is similar to that of a factory, where employees must follow a set of instructions that are both effective and precise. However, some patients with cystic fibrosis (CF) have a mutation in one of their genes, resulting in unclear instructions for manufacturing the protein CFTR.
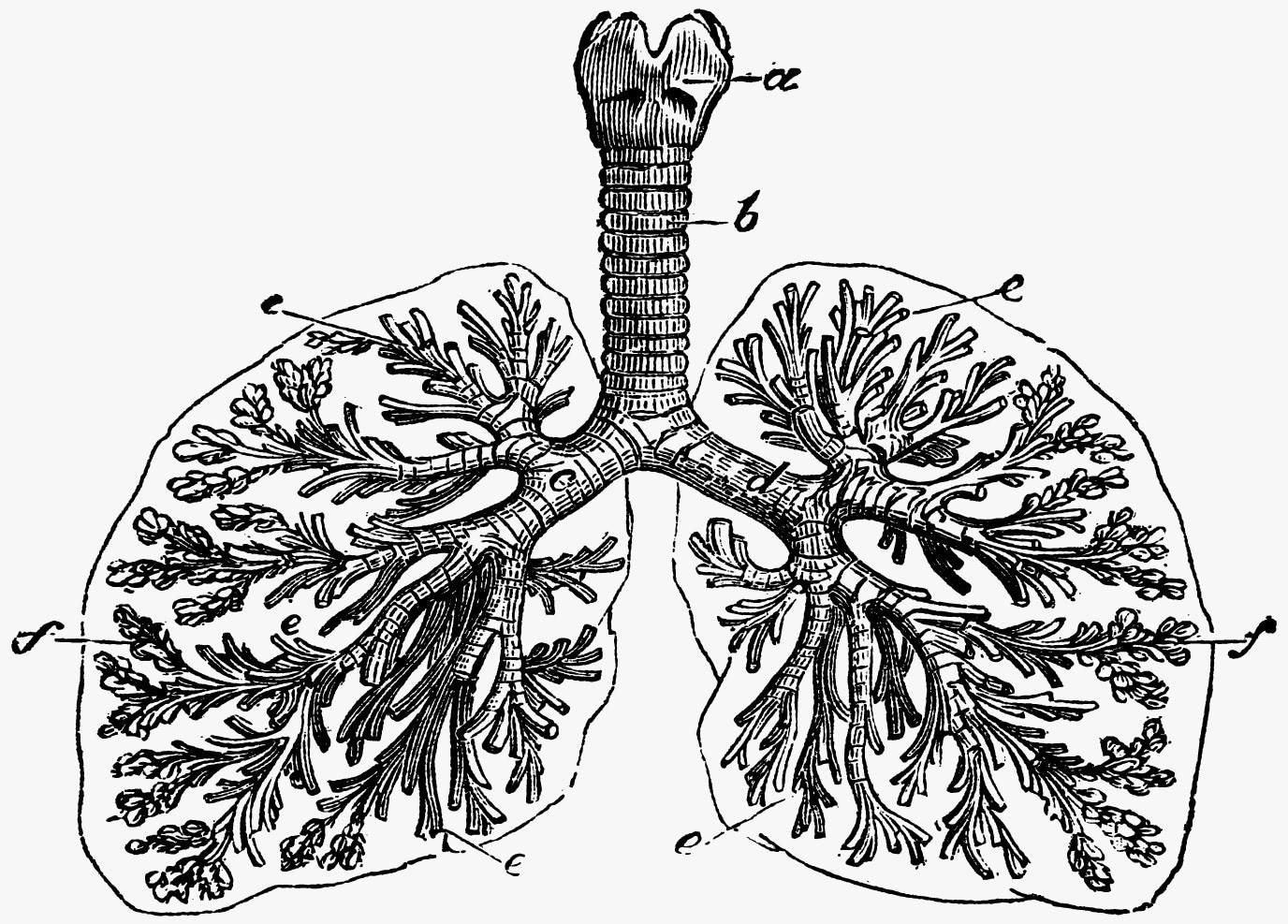
Cystic fibrosis is a disease of the lungs and other organs. It is caused by a dysfunction of a protein called CFTR (cystic fibrosis transmembrane conductance regulator) that leads to a thick mucus and scarring in the lungs and a lack of ability to use nutrients from food. a: top of the windpipe, including the thyroid and cricoid cartilages b: the windpipe, or trachea c, d: major airway branches e: small airway branches f: air sacs called alveoli. Image Credit: © Morphart – stock.adobe.com.
The mutation places a stop sign in the incorrect spot, causing cells to produce very little or no protein. Patients with CFTR deficiency have thick mucus in their lungs and have trouble receiving nourishment from meals.
Professor Adrian Krainer of Cold Spring Harbor Laboratory and his former graduate student Young Jin Kim, an MD, Ph.D. student at Stony Brook University, have discovered a way to change the message containing the CFTR instruction, causing the protein-making machinery to skip over the “stop sign” mutation, allowing a functional version of the protein to be produced.
Researchers anticipate that their novel strategy, which involves the use of a specifically engineered RNA therapy, may aid in the development of drugs for CF patients who have this mutation.
There is a significant unmet therapeutic need for patients with this type of mutation. Many mutations in the CFTR gene are not responsive to drugs used to treat CF.”
Young Jin Kim, Stony Brook University
Other efforts have not proven particularly beneficial. Cutting back on procedures that break up messages from genes with undesirable stop signs or encouraging all genes to plow through stop signs has unexpected consequences.
Two antisense oligonucleotides (ASOs) are used in Krainer and Kim’s genetic manipulation, one on either side of the region containing the stop sign mutation. Krainer and Kim created a detour in cultured human bronchial cells with the stop sign mutation, instructing the cell to bypass the incorrect instruction and finish the rest of the protein. The method does not have to work in every cell every time, and the CFTR protein does not have to be perfect.
We can show that this version of the protein has some activity. In cystic fibrosis, there’s been a lot of work, and so there were many reasons to believe that you don’t need to go to a hundred percent of the normal protein level, that somewhere between 10% and 30% would be substantially beneficial.”
Adrian Krainer, Professor, Cold Spring Harbor Laboratory
The researchers want to improve the ASO technique for CF systems without limiting itself to the Petri dish. Researchers intend to move on with clinical trials, similar to how the first FDA-approved therapy for the genetic disease SMA (spinal muscular atrophy) was developed.
Source:
Journal reference:
Kim, Y. J., et al., (2022) Exon-skipping antisense oligonucleotides for cystic fibrosis therapy. Proceedings of the National Academy of Sciences. doi.org/10.1073/pnas.2114858118