Amyloidosis is a term used to describe a group of diseases defined by the formation of insoluble proteins called amyloids outside of cells as a result of misfolding and aggregation of soluble proteins. Depositions like this cause cellular dysfunction in Alzheimer’s patients, Parkinson’s patients, and dementia patients.
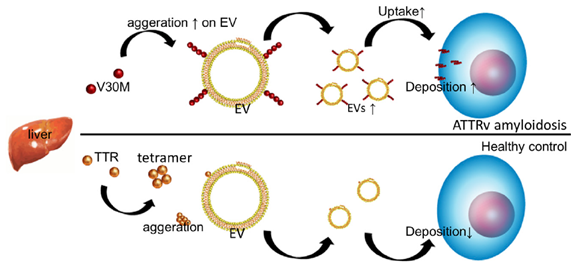 Model of EV-mediated V30M-TTR cell deposition in ATTRv amyloidosis patients, compared to processes in healthy controls. Image Credit: Kanazawa University.
Model of EV-mediated V30M-TTR cell deposition in ATTRv amyloidosis patients, compared to processes in healthy controls. Image Credit: Kanazawa University.
Variants of the transthyretin (TTR) gene cause inherited (variant) transthyretin amyloidosis (abbreviated ATTRv amyloidosis), which causes TTR amyloid deposits in various organs and symptoms such as muscular weakness and cardiac failure.
Extracellular vesicles (EVs)—tiny “biocontainers” contained by a membrane—are known to facilitate the removal of amyloid proteins, but it is unclear if EVs are involved in the synthesis and subsequent accumulation of TTR amyloids in ATTRv amyloidosis.
Rikinari Hanayama and coworkers from Kanazawa University have recently investigated the link between ATTRv amyloidosis and EVs, concluding that the latter plays a key role in TTR amyloid aggregation and deposition.
The researchers initially found some evidence of TTR amyloid in the serum of ATTRv amyloidosis patients. (Serum is the blood that has been stripped of clotting components.) TTR is identified in EVs formed from serum, and the so-called V30M mutation variant of TTR accumulates in the membranes of serum-derived EVs, according to the researchers.
Hanayama and coworkers then investigated what occurred when V30M-TTR amyloids were mixed with serum-derived EVs in cell cultures. Scientists discovered that when serum-derived EVs are present, V30M-TTR amyloid clumps are deposited on cells in a much more spectacular manner, indicating that serum-derived EVs enhance V30M-TTR aggregation and deposition on cells.
The scientists discovered that ATTRv amyloidosis is related to a reduced number of TTR aggregates in serum-derived EVs when they compared ATTRv amyloidosis patients to healthy people. The results suggest that the presence of V30M-TTR and EVs in ATTRv amyloidosis patients leads to a self-enhancing uptake of EVs, which leads to an increased deposition of TTR aggregates in tissue and a reduction in TTR aggregates in serum.
Hanayama and coworkers’ findings imply that TTR in serum-derived EVs might be a target for ATTRv amyloidosis diagnosis and treatment. Because TTR reduces the nucleation of amyloid-β aggregate and its aggregation is increased in EVs, the researchers believe their findings have implications for the knowledge of Alzheimer’s disease.
Researchers conclude “it is necessary to further analyze the relationship between TTR and […] EVs derived from the brain, in addition to EVs derived from the liver and other blood sources.”
Source:
Journal reference:
Yamaguchi, H., et al. (2022) Extracellular Vesicles Contribute to the Metabolism of Transthyretin Amyloid in Hereditary Transthyretin Amyloidosis. Frontiers in Molecular Biosciences. doi.org/10.3389/fmolb.2022.839917.