New study reveals that a family of proteins that help many different types of cells move and retain their form may induce disease when they work too hard and disturb the cellular environment.
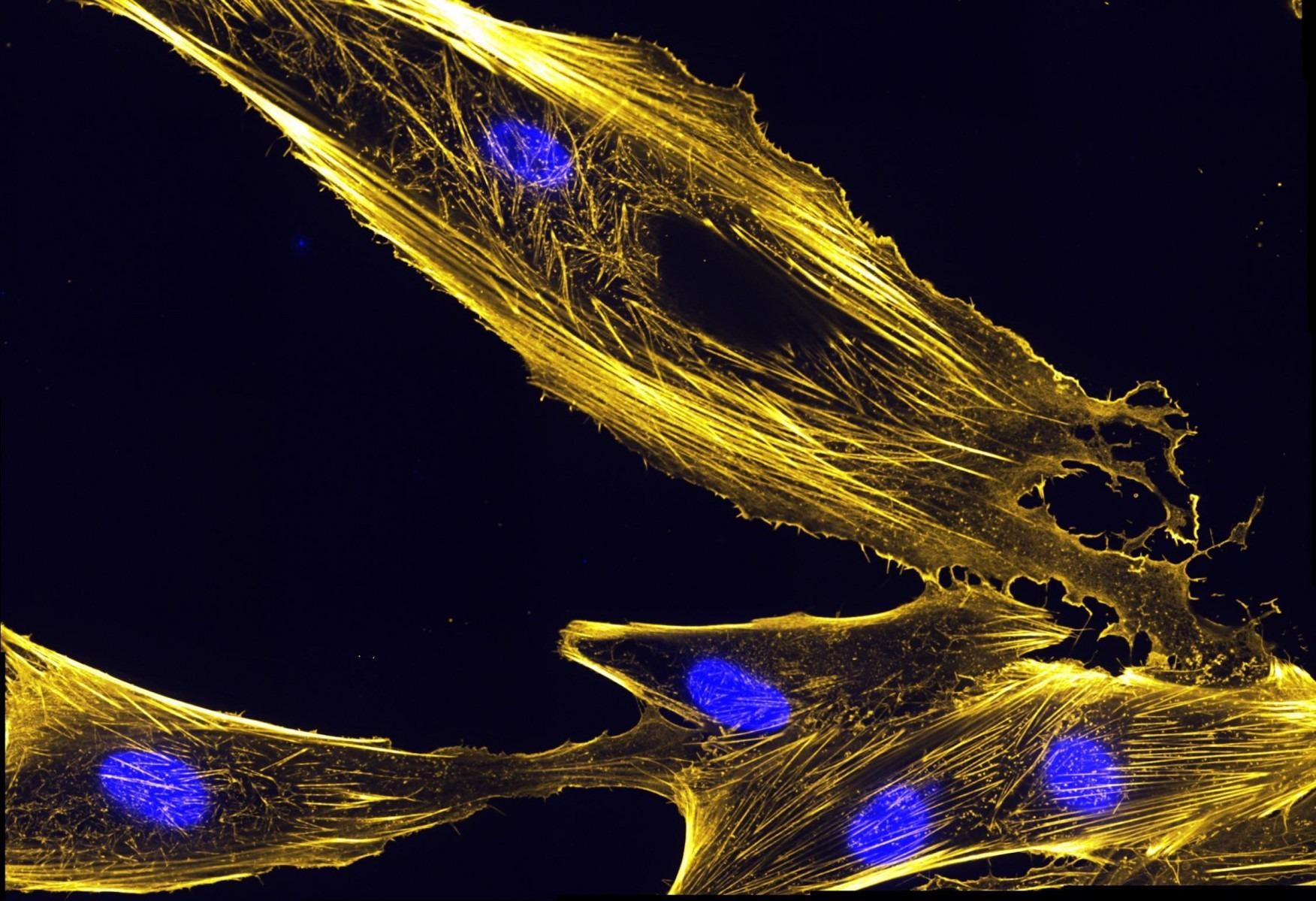 Plastin is a protein that assembles actin bundles, like those pictured here in muscle precursor cells called myoblasts. Image Credit: National Institutes of Health.
Plastin is a protein that assembles actin bundles, like those pictured here in muscle precursor cells called myoblasts. Image Credit: National Institutes of Health.
The research adds to the knowledge of plastins, which bind to and bundle other proteins, which may be regarded as the bones and muscles of cells. Understanding what causes plastin activity might help explain the protein family’s linkages to diseases including cancer, congenital osteoporosis, and spinal muscular atrophy, which are sometimes beneficial and sometimes harmful.
The findings concerning plastin behavior were presented in terms of work-life balance by the researchers. When the protein’s two major segments are in their “at-home” state, they are tightly bonded to each other, but when their bundling tasks in the cellular “workplace” rise—such as when a cell begins to migrate—they can be pushed to disengage from one other.
The study found that plastins continue to undertake aggressive bundling activities even when it is no longer needed when their structure changes unexpectedly.
The study revealed that at least one mistimed enzyme activity may be contributing to the problem, but additional research is needed to completely comprehend the mechanics underlying the changeover between “workaholism” and “weekend” modes.
We need to know this information so we can figure out how to regulate plastins. Because plastins are involved in disease, we see the manifestation of that, but we don’t know how precisely mutations lead to disease. In cancer, or certain autoimmune reactions, knowing exactly what plastins do and how to control their activity could be highly beneficial. If we could manage to inhibit this protein in cancer, it’s likely the cells would become less invasive.”
Dmitri Kudryashov, Study Senior Author and Associate Professor, Chemistry and Biochemistry, The Ohio State University
The work was published in the journal Nature Structural & Molecular Biology (May 19th, 2022).
Plastins are highly conserved, which means they have been found in a wide range of species, from yeast to humans, and have the same function. Although crystal structures for plastins in yeast and plants have been determined, this is one of the first studies to use a combination of cell biology, biochemistry, and cryo-electron microscopy to characterize the structures and functions of human plastins.
Plastin 1, 2, and 3 are the three members of the family, and while they influence various cells in the body, their overall behavior is thought to be constant.
Actins are proteins that assist cells in uniting their contents, maintaining their form, dividing, and migrating. To be more effective, actins must be grouped when they are organized in a thread-like, or filament, shape in a cell. Plastin is a protein that binds actin together.
Plastins have two identical binding sites on their surface, each of which may attach to actin filaments. However, these binding domains have a significant attraction for one another, and when securely bound together, they can only link to actins weakly.
When a group of plastins is weakly linked at many points along the filament, this method of connecting is adequate, and it also allows for plastin recycling across different cellular regions. Plastin can achieve a work-life balance that prioritizes “family” time in this mode.
Actins being pushed in the path of cell movement are situated in a less organized way under certain conditions, particularly when a cell begins to migrate, requiring a strong singular connection to plastin—meaning plastin’s binding domains must disengage from each other to frame that stronger bond to actins. The exact variables that cause this disengagement are unknown.
The same protein can transition from one mode to another depending on the needs of the cell.”
Dmitri Kudryashov, Study Senior Author and Associate Professor, Chemistry and Biochemistry, The Ohio State University
Actins that move away from the cell’s leading edge eventually do not require a strong plastin connection, and plastin that has wandered toward the cell’s center reverts to its self-engaged “weekend” shape and is recycled back to the head of the line to bundle actins there.
The researchers created a mutation in plastin that resembles a molecular alteration found in cancer cells. This modification delayed the disengagement of plastin binding sites, and plastins were not recycled; instead, they kept trying to bundle actins that did not need to be bundled. These findings imply that plastin’s failure to respond to the cell’s requirements may have unfavorable consequences.
This is why it’s important to be engaged in different modes—because the situation in the cell changes.”
Elena Kudryashova, Study Co-Author and Research Scientist, Chemistry and Biochemistry, The Ohio State University
Plastin 2 was used to make these observations. Future research will look into whether plastins 1 and 3 work in the same way, as well as if and how plastin’s “co-workers” in cells play a role in regulating the protein’s actions.
Source:
Journal reference:
Schwebach, C. L., et al. (2022) Allosteric regulation controls actin-bundling properties of human plastins. Nature Structural & Molecular Biology. doi.org/10.1038/s41594-022-00771-1.